Research Highlights
The injectable VitroGel provides a viable in vivo scaffold for novel maternal-fetal adipose stem cell transplant studies
Maternally derived adipose stem cells have emerged as a possible solution to the common immunological rejection observed in fetal repair surgeries.
Institution:

Showa University School of Medicine
Team:
Akihiro Kawashima*, Rika Yasuhara, Ryosuke Akino, Kenji Mishima, Michiko Nasu, Akihiko Sekizawa
Application:
In vivo utilization of VitroGel RGD to study neuronal differentiation capacity
Disease model:
Neurometabolic disorders
Cell types:
PDGFRα+Sca-1+ ADSC
Hydrogel:
VitroGel® RGD (TWG003)
The continuing advancement of genetic testing capabilities has allowed for the earlier diagnosis of congenital birth defects in the fetus, promoting the development of fetal reconstructive surgeries that can correct defects, including brain anomalies, in utero. Among these treatments, stem cell therapies have currently shown promise in the treatment of neurometabolic disorders. However, though the fetus was previously thought to be immune-privileged, emerging studies have confirmed the presence of circulating maternal T-cells, increasing the risk of graft rejection. Therefore, more recent studies searching for a solution to this have turned to mesenchymal stem cells, which have been demonstrated over the last two decades to exhibit a reduced immune response and high plasticity.
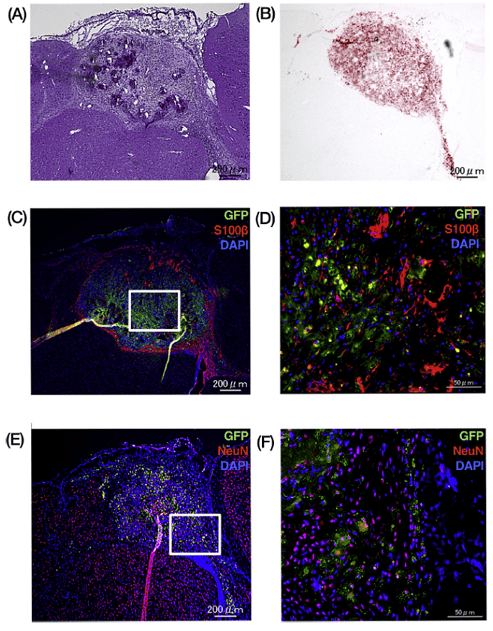
In a novel proof-of-concept study, Akihiro Kawashima and colleagues took this one step further: taking into consideration that the immune response in the fetus comes from maternal T-cells, they sought to investigate whether maternal adipose-derived stem cells (ADSCs) would be more well-tolerated during the repair of neurometabolic deficiencies in utero in a mouse model. To do this, they isolated adipose-derived mesenchymal stem cells and purified lineage-unspecified cells via flow cytometry. Following in vitro verification of their neuronal differentiation capacity, mesenchymal stem cells were mixed with injectable VitroGel RGD scaffold and analyzed for their ability to recapitulate neuronal types in vivo. They observed that though some of cells exhibited Oil Red-O staining, suggesting they had differentiated into adipocytes, the majority of the cells expressed S100B, which marks astrocytes, strongly supporting both the neurological differentiation capacity of adipose-derived stem cells and their transplant capacity. Further characterization revealed that, compared to vehicle-only controls (no MSCs), maternal ADSC transplantation resulted in increased activation of pro-inflammatory cytokines, but reduced CD8+ T-cell infiltration and significant increases in regulatory anti-inflammatory cytokines. Finally, they observed that transplants grew and differentiated successfully in the fetus for at least 28 days after transplantation, demonstrating success not only with reduction of immune rejection but applicability and viability of the injectable VitroGel scaffold for in vivo applications.
Because of the unique needs of mesenchymal stem cells during differentiation, including appropriate 3D environment and the effect of mechanical force on their cell fate decisions, VitroGel provides a suitable microenvironment that can facilitate cell survival and proper cell fate choices for cells in this application. Moreover, because cells are isolated from maternal fat, they must be cultured briefly. Because of VitroGel’s unique tunable feature, they can be cultured in vitro with VitroGel, then switched to the injectable format for transplantation, thereby reducing the stress resulting from environmental changes, ultimately helping to maintain the stemness of these cells and resulting in less population variability.
Related Product: