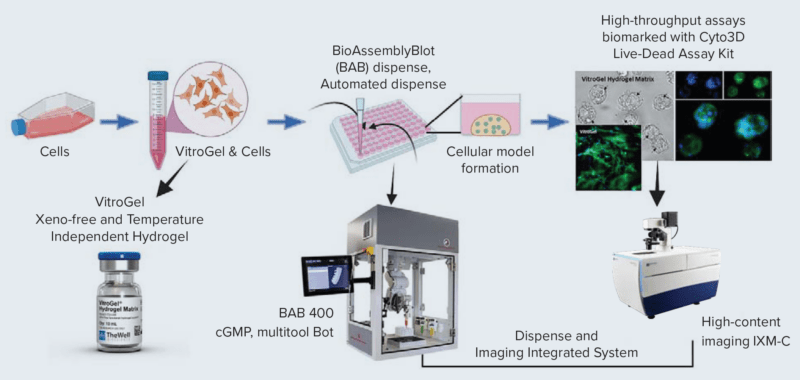
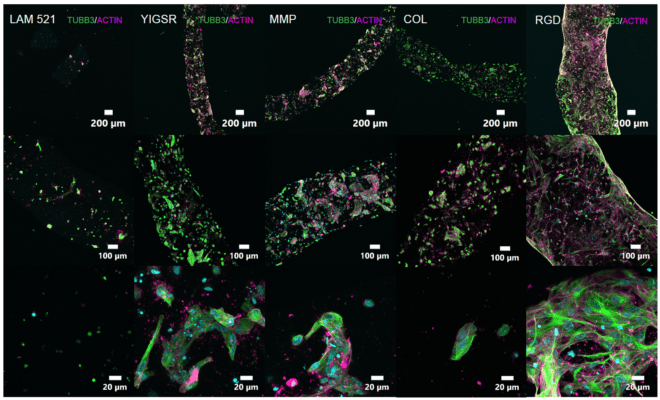
Versatile & Excellent for 3D/2D Cell Culture
Works with cells cultured in hydrogels and animal-based ECM.
Excellent for 3D cell culture.
Cyto3D® Live-Dead Assay Kit
Versatile, live/dead cell viability analysis for 3D and 2D cell culture.
IN STOCK
$238.00
Cyto3D® Live-Dead Assay Kit
Versatile live/dead assay kit for 3D & 2D Culture, Organoids, Spheroids, Stem Cells, Fluorescence Microscopy


Fast & easy setup
No pre-mixing required. Ready-to-use.
Fast one-step staining procedure for cell viability analysis.

Even Staining & Clear Resolution Imaging
No unevenness and no background staining for clearer imaging.

The Cyto3D® Live-Dead Assay Kit is a versatile live/dead assay for 3D and 2D Cell Culture, Organoids, Spheroids, Stem Cells, and Fluorescence Microscopy. It is used to determine the live/dead nucleated cells using a quick one-step staining procedure for analysis on a dual-fluorescence system. The Cyto3D® Live-Dead Assay Kit is recommended for viability analysis of cells/organoids cultured in 3D, 2D coating, and on monolayer and works with cells cultured in animal-based ECMs and other hydrogel systems.
Acridine orange (AO) and propidium iodide (PI), both nuclear staining (nucleic acid binding) dyes, are used in this kit. AO is permeable to live and dead cells and stains all nucleated cells to generate green fluorescence. PI only penetrates the membranes of nucleated cells with compromised membranes and stains the dead cells to generate red fluorescence. Due to the quenching, when cells are stained with both AO and PI, all live nucleated cells fluoresce green and all dead nucleated cells fluoresce red (the PI reduces the fluorescence intensity of the AO by fluorescence resonance energy transfer (FRET)). Non-nucleated materials such as red blood cells, platelets, and debris do not have fluorescence and are ignored by fluorescence microscopes.
Dual-fluorescence viability, using AO and PI, is the recommended viability analysis method for cell lines, primary cells, and stem cells.
Easy setup and use
Specifications
| Formulation | Premixed acridine orange (AO) and propidium iodide (PI), nuclear staining dyes |
| Use | Live dead cell viability analysis for 3D and 2D cell culture |
| Detection Method | Fluorescent |
| Excitation/Emission: | AO (494/517nm), PI (535/617nm) |
| Standard filters | AO (GFP), PI (Texas Red) |
| For use with (Equipment): | Fluorescence microscope, flow cytometer, microplate reader, fluorescence cell counter. |
| Storage | 2 to 8°C (Protect from light) |
| Shipping Conditions: | Ships at ambient temperature |
| Sizes | 1 mL |
| Number of reactions | 500 (at 2 µL per 100 µL) |
Protocols and Resources
Data and References
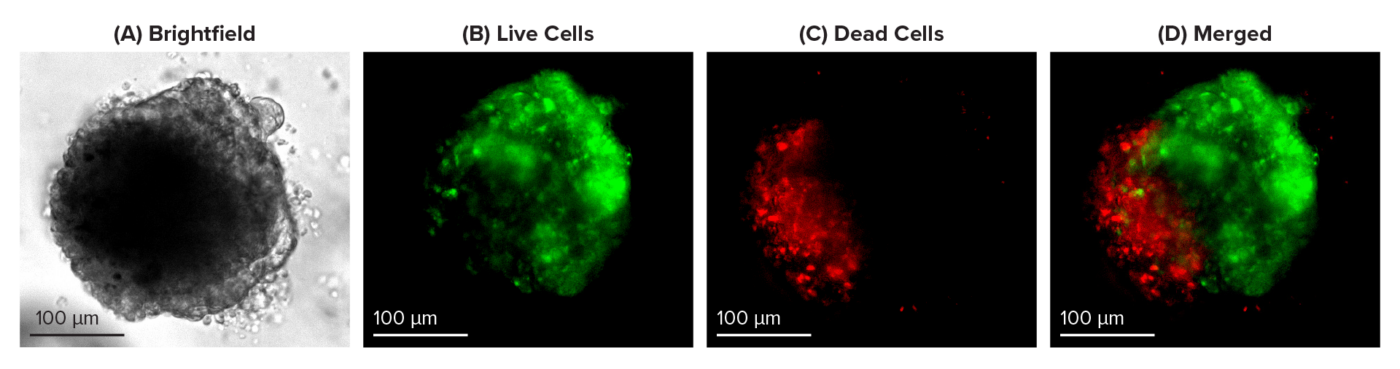
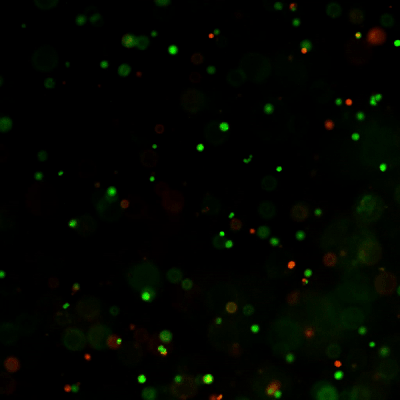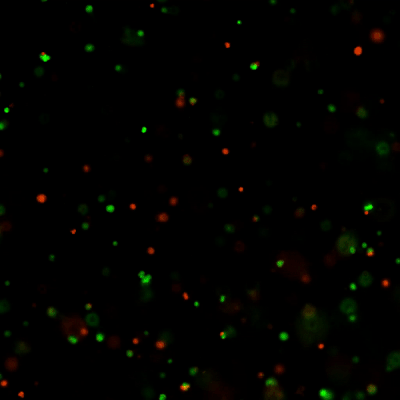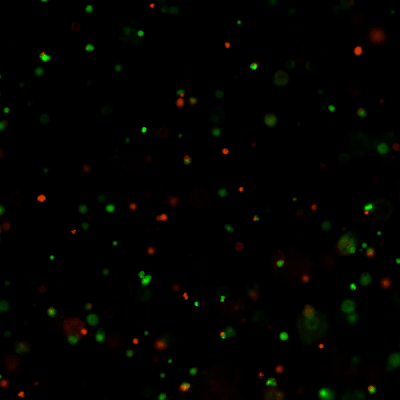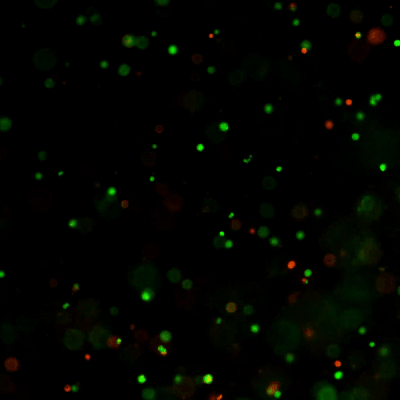
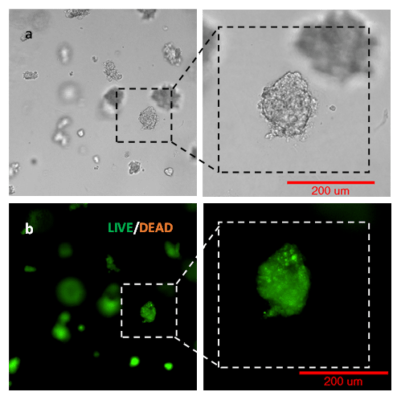
Figure 1: Live-dead cell viability images: Intestinal organoids stained with Cyto3D® Live-Dead Assay Kit.
Intestinal organoids were cultured in regulated conditions for 5 days. Six microliters of Cyto3D® reagent were mixed with organoid culture media (each well includes 150 µL of organoid culture media and 150 µL of hydrogel volume). The mixture was incubated at 37°C for 10-15 min, and the cells were observed under a fluorescence microscope. (A) A bright field image of a mature intestinal organoid. Images show live cells (B: Green) and dead cells (C: Red) in a mature intestinal organoid.
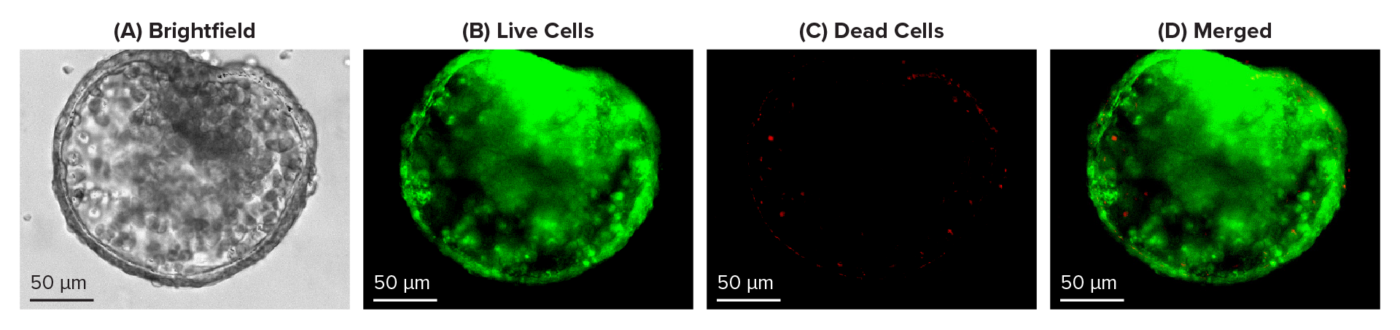
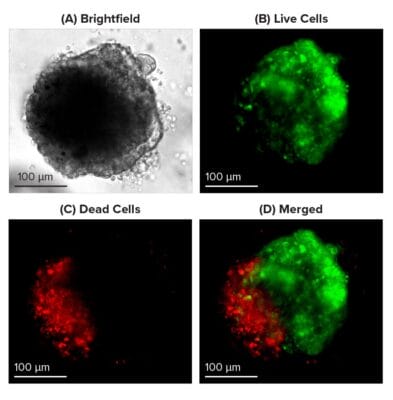
Figure 2: Live-dead cell viability images: Intestinal organoids stained with Cyto3D® Live-Dead Assay Kit.
Intestinal organoids were cultured in regulated conditions for 2-3 days. Six microliters of Cyto3D® Live-Dead Assay reagent were mixed with organoid culture media (each well includes 150 µL of organoid culture media and 150 µL of hydrogel volume). The mixture was incubated at 37°C for 10-15 min, and the cells were observed under a fluorescence microscope. (A) A brightfield image of a young healthy intestinal organoid. Images show live cells (B: Green) and dead cells (C: Red) in a healthy intestinal organoid.
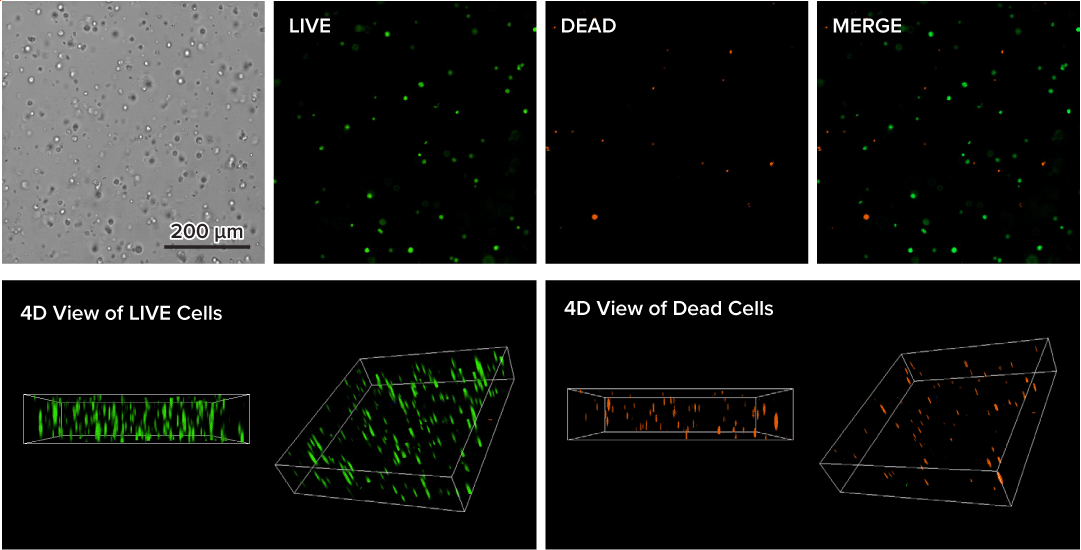
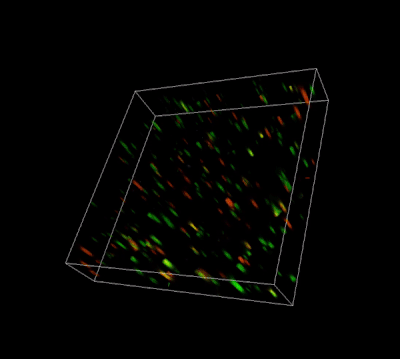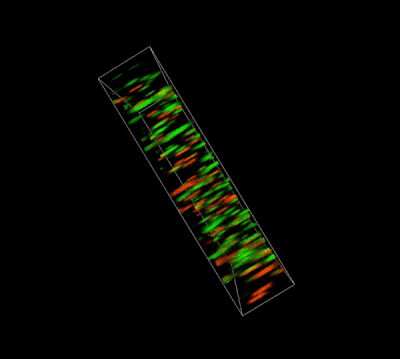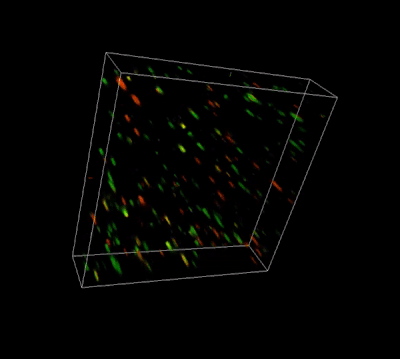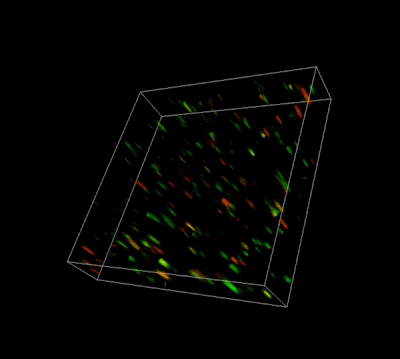
Figure 3. Live-dead cell viability analysis by using Cyto3D® Live-Dead Assay Kit.
Glioblastoma cells (SF 298, about 60% cell viability) were 3D cultured in VitroGel® system for 2 days. Two microliters (2 µL) of Cyto3D® Live-Dead Assay reagent was added to each well containing 50 µL hydrogel and 50 µL cover medium. The mixture was incubated at 37°C for 5-10 min. The cells were then observed under a fluorescence microscope. The images show the Live (green) and Dead (orange) cells within the 3D hydrogel matrix. The z-stack images of cells within hydrogel were then 3D reconstructed and shown in the 4D view images. The live and dead cells at higher levels of the hydrogel are clearly shown in the images using the Cyto3D® Live-Dead Assay Kit.
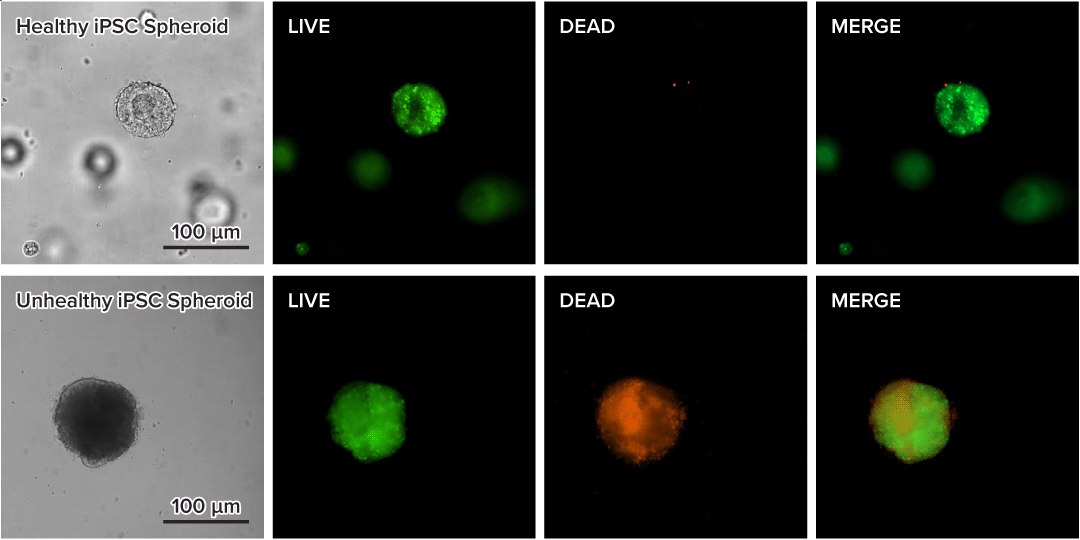
Figure 4. Live-dead cell viability images of stem cell spheroids.
Stem cells were static suspension-cultured in VitroGel® STEM (CAT# VHM02) for 5 days. Two microliters (2 µL) of Cyto3D® Live-Dead Assay reagent was added to each well containing 100 µL cell suspension. The mixture was incubated at 37°C for 5-10 min. The cells were then observed under a fluorescence microscope. The images show the Live (green) and Dead (orange) stem cell spheroids cultured in a 3D hydrogel matrix. The live-dead dyes of Cyto3D® Live-Dead Assay Kit can successfully penetrate into large cell spheroids for cell viability analysis.
Resources using Cyto3D® Live-Dead Assay Kit
Research Highlights | Application Notes
References/Publications
- Lee, H. J., Lau, L. N., Sidhu, S. K., Park, J.-Y., & Yeo, I.-S. L. (2025). Three-Dimensional Hydrogel Culture Reveals Novel Differentiation Potential of Human Bone Marrow-Derived Stem Cells. Prosthesis, 7(3), 52. https://doi.org/10.3390/prosthesis7030052
- Díaz Méndez, A. B., Di Giuliani, M., Sacconi, A., Tremante, E., Lulli, V., Di Martile, M., Vari, G., De Bacco, F., Boccaccio, C., Regazzo, G., & Rizzo, M. G. (2025). Androgen receptor inhibition sensitizes glioblastoma stem cells to temozolomide by the miR-1/miR-26a-1/miR-487b signature mediated WT1 and FOXA1 silencing. Cell Death Discovery, 11(1). https://doi.org/10.1038/s41420-025-02517-6
- Lee, E.-O., Joo, H. K., Yoo, H. J., Kim, C.-S., & Jeon, B. H. (2025). Aspirin-induced acetylation of APE1/Ref-1 enhances RAGE binding and promotes apoptosis in ovarian cancer cells. Korean Journal of Physiology and Pharmacology. https://doi.org/10.4196/kjpp.24.273
- DePalma, T., Rodriguez, M., Kollin, L., Hughes, K., Jones, K., Stagner, E., Venere, M., & Skardal, A. (2025). A Microfluidic Blood Brain Barrier Model to Study the Influence of Glioblastoma Tumor Cells on BBB Function. Small. https://doi.org/10.1002/smll.202411361
- Kim, M. S., & Kim, M. S. (2025). Deubiquitination of epidermal growth factor receptor by ubiquitin-specific peptidase 54 enhances drug sensitivity to gefitinib in gefitinib-resistant non-small cell lung cancer cells. PLoS ONE, 20(4), e0320668–e0320668. https://doi.org/10.1371/journal.pone.0320668
- Patel, D. P. (2025). Developing and Validating Brain Tumor Organoid Models to Evaluate Novel Therapeutics In Vitro. Scholar Commons. https://scholarcommons.sc.edu/senior_theses/741/
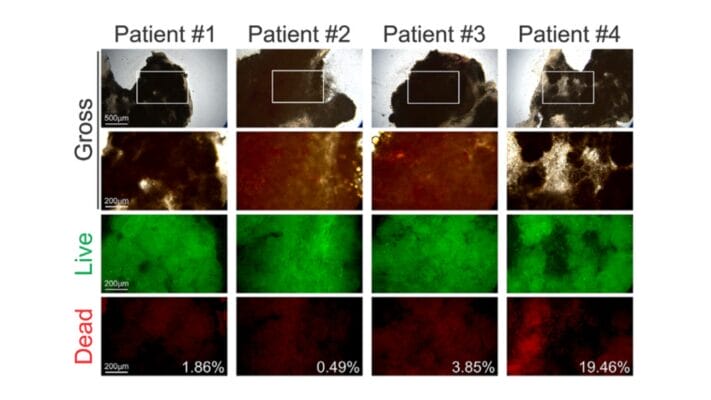
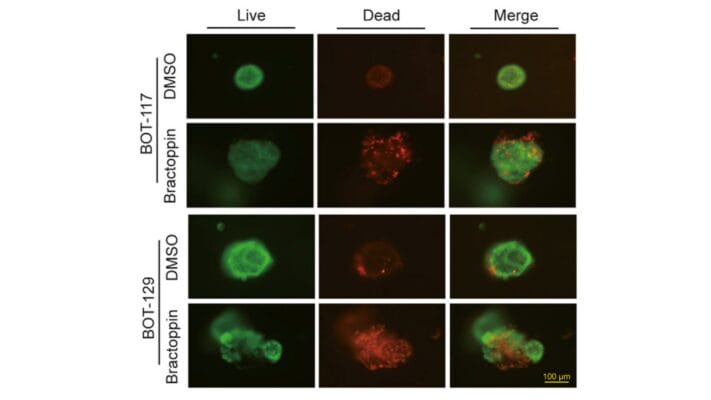
- Chang, W.-H., Chin, A. I., & Chen, C.-H. (2025). Protocol for a patient-derived preclinical platform to model tumor-immune interactions and evaluate therapeutic efficacy. STAR Protocols, 6(1), 103623–103623. https://doi.org/10.1016/j.xpro.2025.103623
- Donato, M. D., Cristiani, C. M., Capone, M., Garofalo, C., Madonna, G., Passacatini, L. C., Ottaviano, M., Paolo Antonio Ascierto, Auricchio, F., Carbone, E., Migliaccio, A., & Castoria, G. (2025). Role of the androgen receptor in melanoma aggressiveness. Cell Death and Disease, 16(1). https://doi.org/10.1038/s41419-025-07350-4
- Kawai-Kawachi, A., Lenormand, M. M., Astier, C., Herbel, N., Cutrona, M. B., Ngo, C., Garrido, M., Eychenne, T., Dorvault, N., Bordelet, L., Song, F. F., Bouyakoub, R., Loktev, A., Romo-Morales, A., Henon, C., Colmet-Daage, L., Vibert, J., Drac, M., Brough, R., & Schwob, E. (2025). Replication Stress is an Actionable Genetic Vulnerability in Desmoplastic Small Round Cell Tumors. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-23-3603
- Kim, K., Lee, Y., Jung, K. B., Kim, Y., Jang, E., Lee, M., Son, M., & Lee, H. J. (2024). Highly Stretchable 3D Microelectrode Array for Noninvasive Functional Evaluation of Cardiac Spheroids and Midbrain Organoids. Advanced Materials. https://doi.org/10.1002/adma.202412953
- Ossi Arasalo, Lehtonen, A. J., Kielosto, M., Heinonen, M., & Juho Pokki. (2024). Probabilistic analysis of spatial viscoelastic cues in 3D cell culture using magnetic microrheometry. Biophysical Journal. https://doi.org/10.1016/j.bpj.2024.12.010
- Motawea, A., Maria, S. N., Maria, D. N., Jablonski, M. M., & Mohamed Moustafa Ibrahim. (2024). Genistein transfersome-embedded topical delivery system for skin melanoma treatment: in vitro and ex vivo evaluations. Drug Delivery, 31(1). https://doi.org/10.1080/10717544.2024.2372277
- Meng, H., Miao, H., Zhang, Y., Chen, T., Yuan, L., Wan, Y., Jiang , Y., Zhang , L., & Cheng , W. (2024). YBX1 promotes homologous recombination and resistance to platinum-induced stress in ovarian cancer by recognizing m5C modification. Cancer Letters, 217064–217064. https://doi.org/10.1016/j.canlet.2024.217064
- Noriyuki Imazu, Takehiro Torisu, Akihito Yokote, Junji Umeno, Keisuke Kawasaki, Shin Fujioka, Yuichi Matsuno, Tomohiro Nagasue, Shinichiro Kawatoko, Tomohiko Moriyama, Tomoki Nitahata, Yushi Uchida, Seishi Aihara, Yoshiaki Taniguchi, Yoshinao Oda, & Takanari Kitazono. (2024). Arginase 2 attenuates ulcerative colitis by antioxidant effects of spermidine. Journal of Gastroenterology. https://doi.org/10.1007/s00535-024-02104-z
- Cheng, W.-M., Li, P.-C., Minh Tran-Binh Nguyen, Lin, Y.-T., Huang, Y.-T., Cheng, T.-S., Nguyen, T.-H., Tran, T.-H., Huang, T.-Y., Hoang, T.-H., Chen, S.-Y., Chu, Y.-C., Wu, C.-W., Lee, M.-F., Chiou, Y.-S., Liu, H.-S., Hong, Y.-R., Peter Mu-Hsin Chang, Hu, Y., & Chang, Y.-C. (2024). Repurposing pitavastatin and atorvastatin to overcome chemoresistance of metastatic colorectal cancer under high glucose conditions. Research Square (Research Square). https://www.researchsquare.com/article/rs-4218809/latest
- Badawe, H., Harouz, J., Raad, P., Abu, K., Freije, Anthony., Ghali, K., Abou-Kheir, W., Khraiche, M. (2024). Experimental and Computational Analysis of High-Intensity Focused Ultrasound Thermal Ablation in Breast Cancer Cells: Monolayers vs. Spheroids. Cancers 2024, 16(7), 1274; https://doi.org/10.3390/cancers16071274
- Wang, H., Zhang, Y., Miao, H., Xu, T., Nie, X., & Cheng, W. (2024). CircRAD23B promotes proliferation and carboplatin resistance in ovarian cancer cell lines and organoids. Cancer Cell International, 24(1). https://doi.org/10.1186/s12935-024-03228-1
- Miao, H., Meng, H., Zhang, Y., Chen, T., Zhang, L., & Cheng, W. (2024). FSP1 inhibition enhances olaparib sensitivity in BRCA-proficient ovarian cancer patients via a nonferroptosis mechanism. Cell Death & Differentiation, 1–14. https://doi.org/10.1038/s41418-024-01263-z
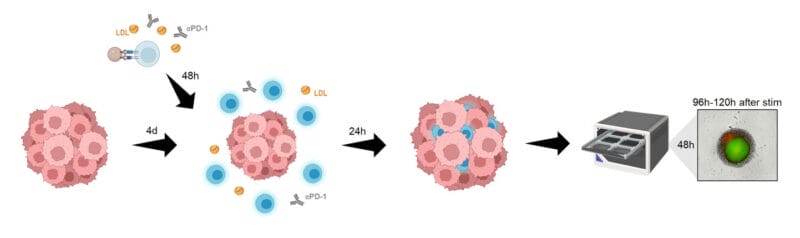
- Babl, N., Hofbauer, J., Matos, C., Voll, F., Ayse Nur Menevse, Rechenmacher, M., Mair, R., Philipp Beckhove, Herr, W., Siska, P. J., Renner, K., Kreutz, M., & Schnell, A. (2023). Low-density lipoprotein balances T cell metabolism and enhances response to anti-PD-1 blockade in a HCT116 spheroid model. Frontiers in Oncology, 13. https://doi.org/10.3389/fonc.2023.1107484
- Belén, A., Sacconi, A., Tremante, E., Lulli, V., Caprara, V., Rosanò, L., Goeman, F., Carosi, M., Marta Di Giuliani, Vari, G., Silvani, A., Pollo, B., Garufi, C., Ramponi, S., Simonetti, G., Ciusani, E., Chiara Mandoj, Stefano Scabini, Villani, V., & Agnese Pò. (2023). A diagnostic circulating miRNA signature as orchestrator of cell invasion via TKS4/TKS5/EFHD2 modulation in human gliomas. Journal of Experimental & Clinical Cancer Research, 42(1). https://doi.org/10.1186/s13046-023-02639-8
- , et al. (2023) MCT4 blockade increases the efficacy of immune checkpoint blockade. https://doi.org/10.1136/jitc-2023-007349
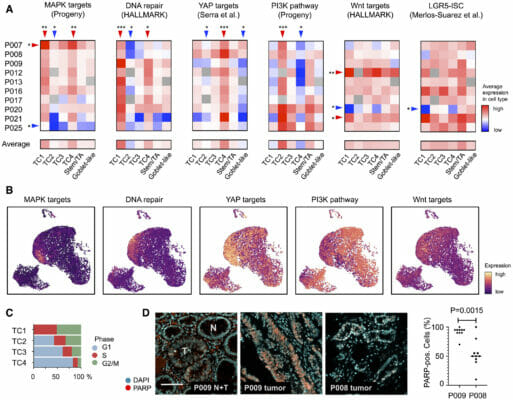
- Uhlitz, F., Bischoff, P., Peidli, S., Sieber, A., Trinks, A., Lüthen, M., Obermayer, B., Blanc, E., Ruchiy, Y., Sell, T., Mamlouk, S., Arsie, R., Wei, T., Klotz‐Noack, K., Schwarz, R. F., Sawitzki, B., Kamphues, C., Beule, D., Landthaler, M., & Sers, C. (2021). Mitogen‐activated protein kinase activity drives cell trajectories in colorectal cancer. EMBO Molecular Medicine, 13(10). https://doi.org/10.15252/emmm.202114123
| Size | 1 mL |
|---|