Application Notes
Xeno-Free Angiogenesis Cell Models Using the VitroGel System
Application Note
TheWell Bioscience, North Brunswick, NJ 08902

Introduction
Angiogenesis, or the process of new blood vessel formation by sprouting from the pre-existing vasculature, plays a significant role in physiological and pathological processes, such as tumor growth or inflammation. The angiogenic microenvironment is critical in developing therapies for these pathologies, as endothelial cells can shift from quiescent to angiogenic states, leading to migration or elongation that can be difficult to detect in the regular 2D culture methods1. Importantly, in vitro models which precisely mimic this extracellular matrix (ECM) with adjustable properties for an in-depth understanding of such microenvironmental conditions are lacking. The current studies of angiogenesis assay rely highly on the native ECM (such as Matrigel), which has non-adjustable hydrogel compositions and properties. Such traditional methods only allow for experiments on growth factors or inhibitors in the media and do not support research concerning how certain properties of the ECM may affect angiogenesis. Thus, there is a great need for reliable human angiogenic models with tunable angiogenic microenvironments to increase our understanding of pathologies related to endothelial cells2,3. Such advanced models can provide novel information on how endothelial cells undergo morphological changes or interact with neighboring cells to impact angiogenesis in response to certain stimuli and increase our understanding of pathophysiologies like Peripheral Vascular Disease, Stroke, Hypertension, and more4.
the pre-existing vasculature, plays a significant role in physiological and pathological processes, such as tumor growth or inflammation. The angiogenic microenvironment is critical in developing therapies for these pathologies, as endothelial cells can shift from quiescent to angiogenic states, leading to migration or elongation that can be difficult to detect in the regular 2D culture methods1. Importantly, in vitro models which precisely mimic this extracellular matrix (ECM) with adjustable properties for an in-depth understanding of such microenvironmental conditions are lacking. The current studies of angiogenesis assay rely highly on the native ECM (such as Matrigel), which has non-adjustable hydrogel compositions and properties. Such traditional methods only allow for experiments on growth factors or inhibitors in the media and do not support research concerning how certain properties of the ECM may affect angiogenesis. Thus, there is a great need for reliable human angiogenic models with tunable angiogenic microenvironments to increase our understanding of pathologies related to endothelial cells2,3. Such advanced models can provide novel information on how endothelial cells undergo morphological changes or interact with neighboring cells to impact angiogenesis in response to certain stimuli and increase our understanding of pathophysiologies like Peripheral Vascular Disease, Stroke, Hypertension, and more4.
The VitroGel® Angiogenesis Assay Kit from TheWell Bioscience is a revolutionary tool for researchers to study the effect of both hydrogel properties and culture media on the angiogenesis process. The xeno-free functional VitroGel system uses tunable hydrogel properties, allowing researchers to study such processes easily, efficiently, and with great control for establishing an in vitro angiogenesis model. This system easily allows for endothelial cell culture to promote tube formation, invasion, or even animal injection via 2D hydrogel coating or 3D culture.
We provide two versions of VitroGel Angiogenesis Assay Kits: 1) ready-to-use VitroGel® Angiogenesis Assay Kit, VHM06-K (VitoGel AAK), which has a fixed hydrogel mechanical strength with different supplements to adjust the growth factors within the hydrogel matrix; and 2) high-concentration VitroGel® Angiogenesis Assay HC Kit, TWG011-K (VitroGel AAK-HC), which has a tunable high concentration hydrogel to allow full control of the hydrogel’s mechanical strength and different supplements to adjust the growth factors within the hydrogel matrix.
In this application note, we use both ready-to-use VitroGel Angiogenesis Assay Kit and high-concentration VitroGel Angiogenesis Assay HC Kit to demonstrate the effects of hydrogel property modification on the angiogenesis assays of human umbilical vein endothelial cells (HUVECs). The experiments were started with a 2D hydrogel coating culture method, where we prepared a thick layer of hydrogel from the VitroGel AAK at the bottom of a cell culture plate and seeded the HUVEC cells on the top of the hydrogel layer. The performances of HUVECs angiogenesis responded significantly on different hydrogel compositions (vascular endothelial growth factor (VEGF) positive, VEGF negative, or no growth factor) even cultured with the same complete endothelial cell culture medium by showing different lumen structures and tube formations. On the other hand, we were able to adjust the hydrogel concentrations by using the VitroGel AAK-HC. The data indicate that besides growth factors within the matrix, the mechanical properties can heavily impact cell viability and tube formation – an influence that was previously unknown due to limitations on traditional experiments which relied on the native ECM. The same observation can be further extended to 3D cell culture when HUVECs were encapsulated within the hydrogel matrix.
Our observations and analyses display the effects of the modifiability and mechanical strength of the hydrogel matrix, along with optimal material concentrations, culture times, and 3D tube structure formation and networking using the VitroGel hydrogel. We demonstrate that growth factors not only present in the medium but in the hydrogel matrix itself significantly impact tube formation. Replacing the native ECM with a xeno-free tunable VitroGel hydrogel system enables researchers to study the relationship between physical, chemical, and/or biological properties of the microenvironment and cell behavior.
In short, both of the VitroGel Angiogenesis Assay Kits are powerful tools that open the door for researchers to explore novel aspects of angiogenesis. We hope these kits inspire individuals and research teams to build complex tissue models that elevate drug screen processes, cell invasion assays, and disease-related research involving endothelial cells and angiogenesis.
Materials and Methods
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were maintained in a complete endothelial growth medium (EGM) with 10% Fetal Bovine Serum (FBS) and a 1x penicillin-streptomycin antibiotic. The cells were passaged when the culture reached 80-90% confluence.
Adjustment of Hydrogel Properties
1) Study the effect of growth factors in hydrogel compositions on angiogenesis assay
To investigate the effects of growth factors within the hydrogel matrix on cell growth and tube formation, HUVECs were used together with the ready-to-use VitroGel AAK kit. Two types of VitroGel AAK supplements can be used to mix with VitroGel AAK hydrogel directly. VitroGel AAK Supplement 1 (does not have VEGF) was used to prepare the VEGF-negative hydrogel, and VitroGel AAK Supplement 2 (contains VEGF) was used to prepare the VEGF-positive hydrogel. To prepare the hydrogel without additional growth factors, we used VitroGel AAK hydrogel to mix with EGM basal medium directly (please read the user protocol of VitroGel AAK Kit for detailed hydrogel preparation).
2) Study the effect of the mechanical strength of hydrogels on angiogenesis assay
The high-concentration VitroGel AAK-HC kit was used to adjust the mechanical strength of hydrogel. The VitroGel AAK-HC hydrogel was mixed with VitroGel AAK Dilution Solution at 1:0, 1:1 and 1:3 (v/v) ratios respectively. The diluted hydrogel mixture was then used to mix with VitroGel AAK supplement to prepare 2D hydrogel coating culture or 3D culture according to the method descriptions below (please read the user protocol of VitroGel AAK-HC Kit for detailed hydrogel preparation).
2D Hydrogel Coating Culture
Cells were harvested using trypsin and then resuspended in complete endothelial growth medium + 10% FBS at a seeding density of 0.5 x 106 cells/mL. Both the VitroGel AAK and VitroGel AAK-HC hydrogels were prepared for cell culture using the following steps (using AAK Supplement 1 as an example).
- Add 1 mL hydrogel solution to 500 µL AAK Supplement 1 and gently pipette up and down 5-10 times to mix thoroughly. Note: Keep VitroGel AAK solution and AAK Supplement 1 at a 2:1 (v/v) mixing ratio.
- Transfer the 50 µL hydrogel mixture to a 96-well plate. Gently swirl the plate to ensure even coverage on the bottom of the wells.
- Wait 10-15 minutes at room temperature for soft gel formation. Do not disturb the hydrogel by shaking the plate during this time.
- Carefully add 50 µL cell suspension to the top of the gel. Incubate at 37°C.
- Incubate the cells at 37°C and change 50-80% of the top medium every other day
3D Culture
For 3D culture of HUVECs, the VitroGel AAK and VitroGel AAK-HC were used to observe tube formation and structure. The hydrogel-cell mixtures were prepared using the following steps (using AAK Supplement 1 as an example):
- Prepare cell suspensions in the AAK Supplement 1. Recommended cell concentration is 1-2 x 106 cells/mL.
- Add 1 mL of hydrogel solution to 500 µL of cell suspension from the previous step. Gently pipette up and down 5-10 times to mix thoroughly, keeping the hydrogel and cell suspension at a 2:1 (w/v) ratio.
- Transfer 50 µL hydrogel mixture to a 96-well plate. Gently swirl the plate to ensure even coverage on the surface of each well.
- Wait 10-15 minutes at room temperature for soft gel formation. Do not disturb the hydrogel by shaking the plate during this time.
- Carefully add 50 µL additional medium on top of the well to cover the hydrogel.
- Place the plate in an incubator at 37°C and change 50-80% of the top medium every other day without disturbing the hydrogel (or change the cover medium according to the experiment design).
Fluorescence Imaging
For fluorescence imaging, the cells were fixed and stained with Actin Green and NucBlue to label F-actin filaments along with the cell nuclei. The following steps were used:
- Carefully remove the cover media from the top of the hydrogel with a pipette.
- The hydrogel was washed with 100 µL of D-PBS. The wash buffer was left to sit on the hydrogel for 1 minute before removal.
- 100 µL of cell fixation solution (4% formaldehyde) was added to the surface of the hydrogel.
- The plate was incubated for 15-30 minutes at room temperature, followed by the removal of the fixation solution.
- The hydrogel was gently washed three times with 100 µL of D-PBS, allowing the wash buffer to sit for 1 minute between washes.
- 100 µL of permeabilization solution (0.1% Triton X-100) was added and incubated for 5 minutes at room temperature.
- The permeabilization solution was carefully removed and the well was washed three times with 100 µL of D-PBS, allowing the wash buffer to sit for 1 minute between washes.
- Add 100 µL of blocking solution (3% BSA in D-PBS) to the hydrogel and incubate for 60 minutes at room temperature.
- The blocking solution was removed and ActinGreenTM 488 or DRAQ5™ ReadyProbesTM reagents (ThermoFisher Scientific) were added according to their manufacturer’s protocol.
- The plate was incubated for 30 minutes, followed by the addition of NucBlueTM Fixed Cell Stain Ready ProbesTM reagent (ThermoFisher Scientific).
- The plate was incubated in the dark for 5 minutes and fluorescence imaging was performed.
Results and Discussion
HUVECs were cultured on the surface of the hydrogel. Figure 1 displays time-lapse images of HUVEC tube formation over 18 hours after seeding on top of either the VitroGel AAK (A) or AAK-HC hydrogels (B) with the VEGF-positive AAK Supplement 2.
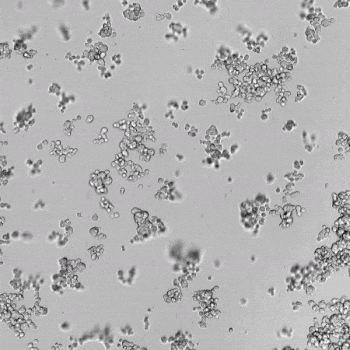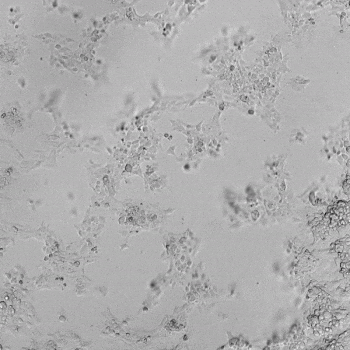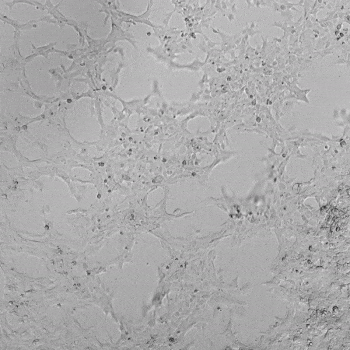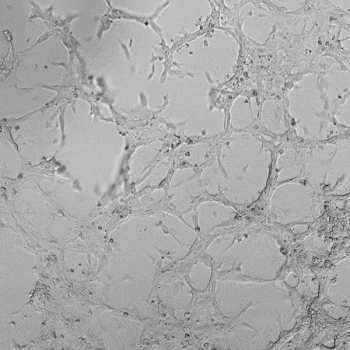
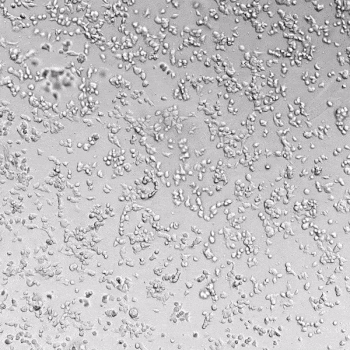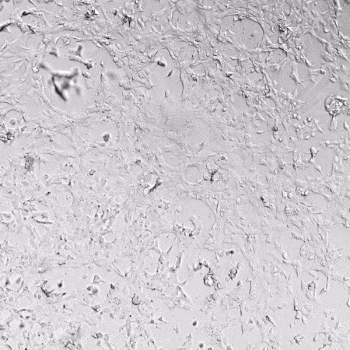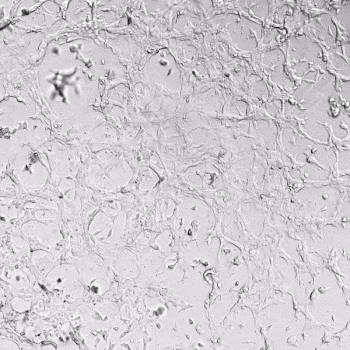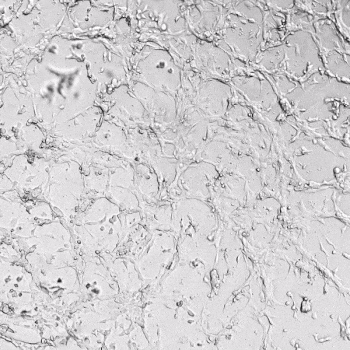
Figure 1. Tube formation of endothelial cells on top of VitroGel AAK hydrogel with tube formation supplement, AAK Supplement 2. Time-lapse video of tube formation during 18 hours after Human Umbilical Vein Endothelial Cells (HUVEC) seeding on top of LEFT) VitroGel AAK hydrogel, or RIGHT) VitroGel AAK-HC hydrogel with AAK Supplement 2.
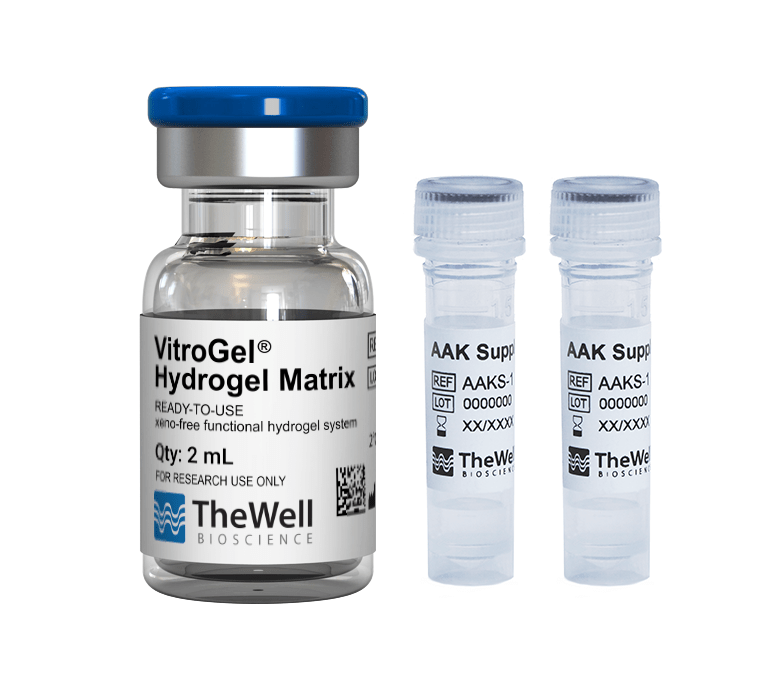
Fluorescence microscopy was performed to visualize HUVEC viability and tube formation on the VitroGel AAK or VitroGel AAK-HC hydrogel. Figure 2 shows immunofluorescence images of actin filament and nucleic acid staining of the HUVECs. Here, we observed an abundance of actin filaments and nucleic acid, indicating the hydrogel’s immense viability along with overall tube formation and morphology.
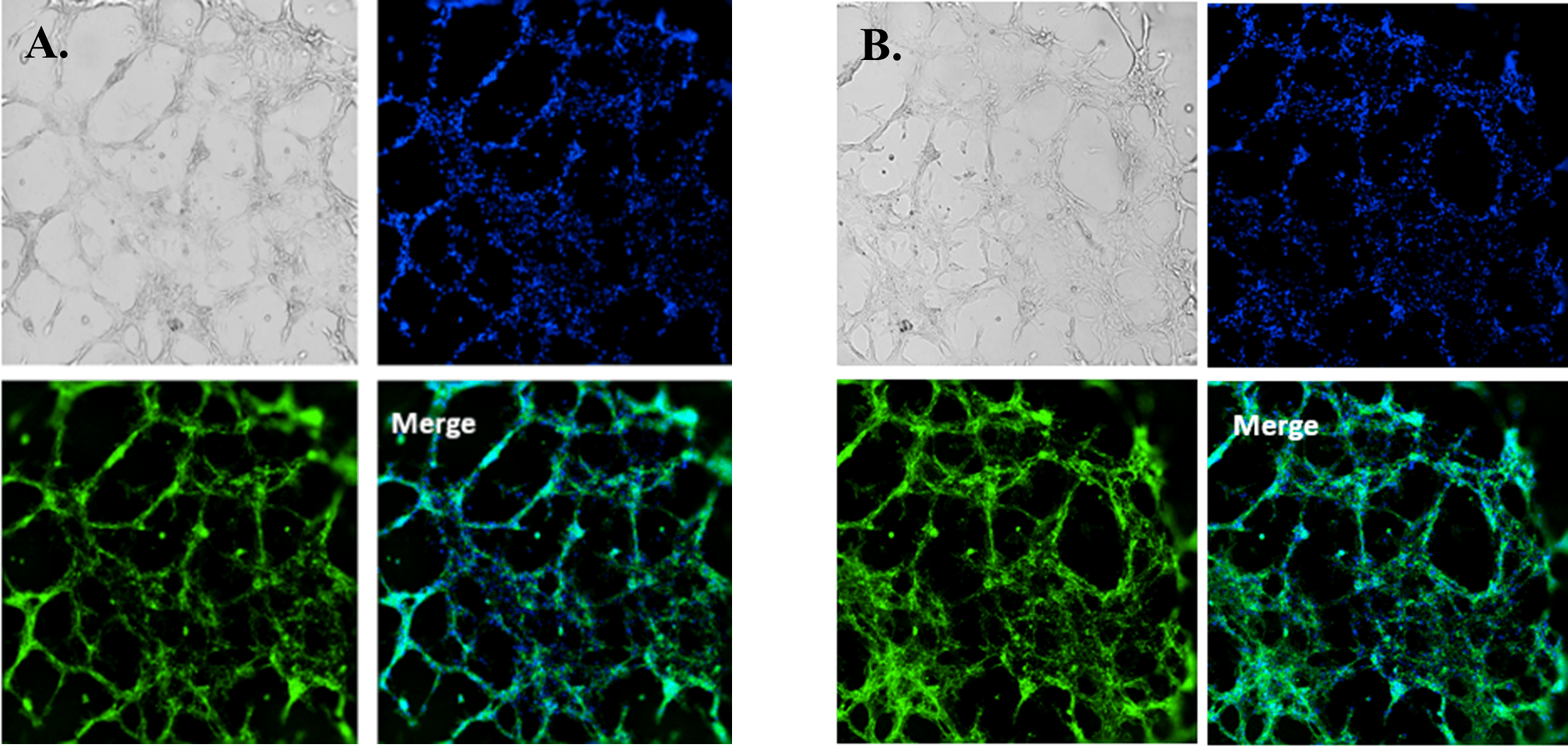
Figure 2. HUVEC cell growth on top of VitroGel AAK hydrogel with cell growth supplement, AAK Supplement 1. Tube morphology of HUVEC cells on top of A) The VitroGel AAK hydrogel or B) the VitroGel AAK-HC hydrogel. The cells were fixed and stained with DAPI (blue) and ActinGreen (green).
When using the hydrogel with VEGF negative supplement (VitroGel AAK Supplement 1), HUVECs were able to attach and grow on the surface without tube formation in both cases of VitroGel AAK and VitroGel AAK-HC hydrogels (Figure 3). Figures 2 and 3 demonstrate that the VEGF growth factors within the hydrogel matrix can dramatically impact tube formation despite the same complete culture medium used in both hydrogel conditions. Figure 3 also illustrates clear cell proliferation with an abundance of DNA in both hydrogel preparations at the VEGF negative condition.
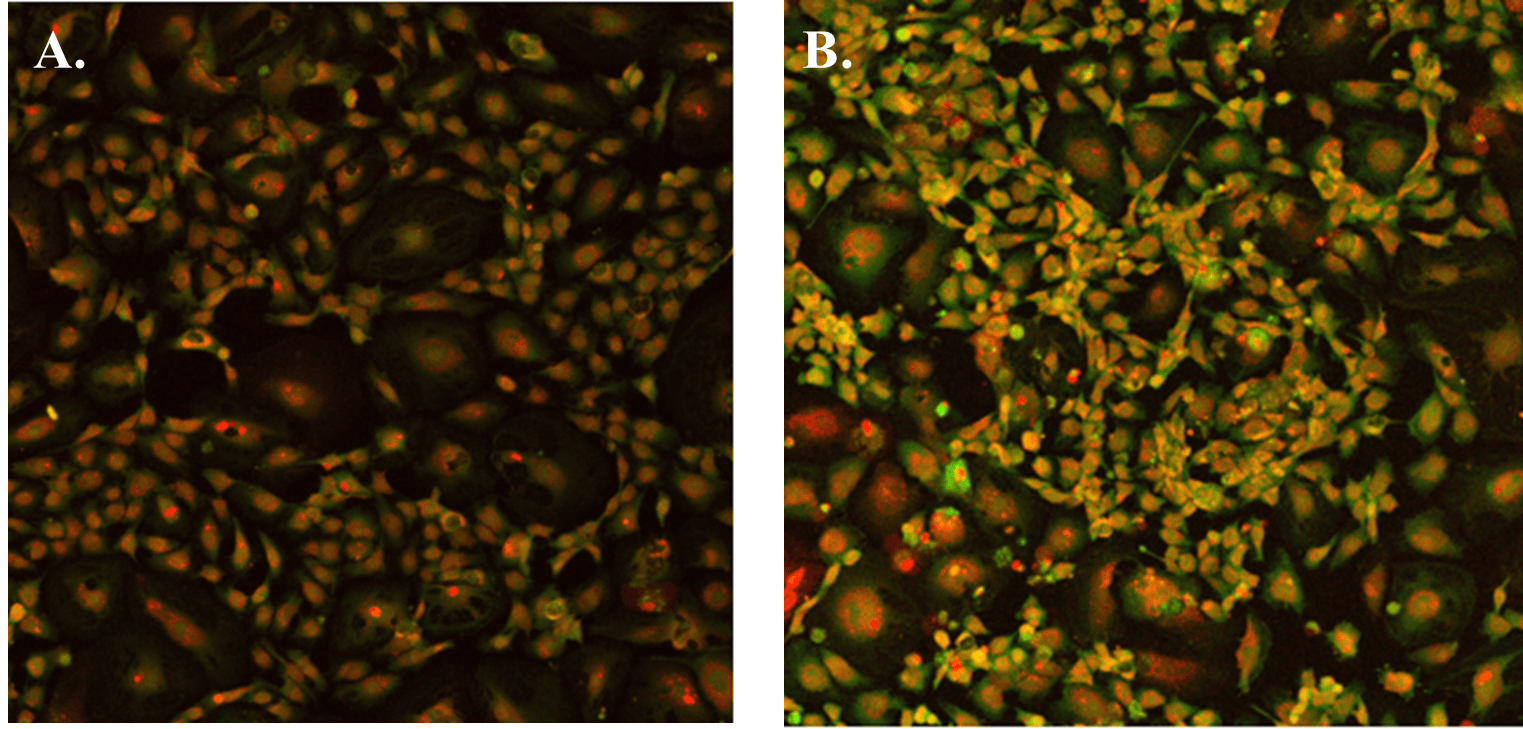
Figure 3. The image above shows HUVEC cells attached and grown on the surface of A) VitroGel AAK hydrogel or B) VitroGel AAK-HC hydrogel with cell growth supplement AAK Supplement 1. The cells were fixed and stained with DRAQ5 (red) and actin green (green).
Next, we aimed to compare HUVEC growth on VitroGel AAK hydrogel that was prepared with or without growth factor supplementation to highlight growth factors’ effects on the hydrogel matrix for angiogenesis. For this experiment, the same complete endothelial growth medium was used on top of the hydrogel for cell culture. HUVEC cell growth on top of hydrogel with VitroGel AAK Supplement 2 or without growth factors was obtained at various time points in Figure 4. Figures 4B and 4D illustrate cells grown on the surface of the hydrogel, which contained no growth factor supplement, while Figures 4A and 4C display images of cells given VitroGel AAK-Supplement 2. Interestingly, although the complete cell culture medium was used in both culture conditions, the time-lapse videos show marked improvements in cell attachment and tube formation in the hydrogel matrix with the addition of cell growth factors when compared to the cells not receiving growth factor supplement from the hydrogel matrix. The images provide further evidence for the benefit of supplements from hydrogel matrix on HUVEC proliferation and viability, as cells without growth factor supplementation clearly display fewer lumen structures (red arrows) compared to those receiving growth factors support from hydrogel matrix.
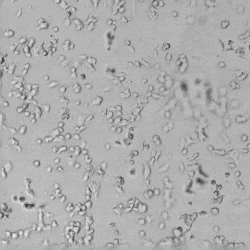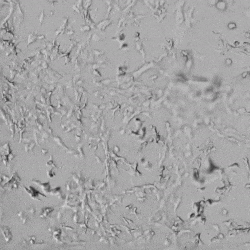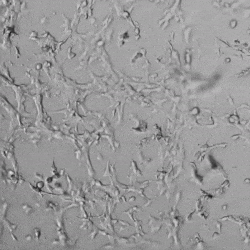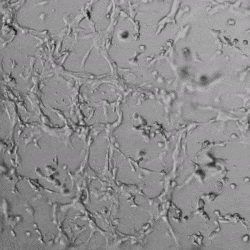
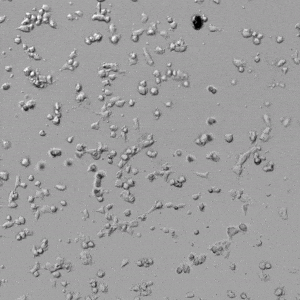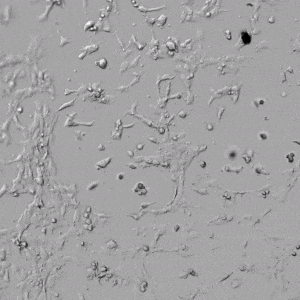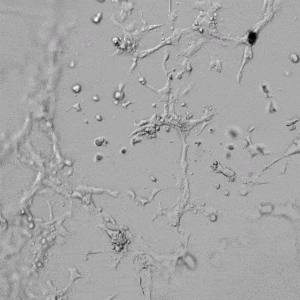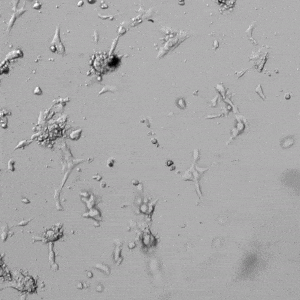
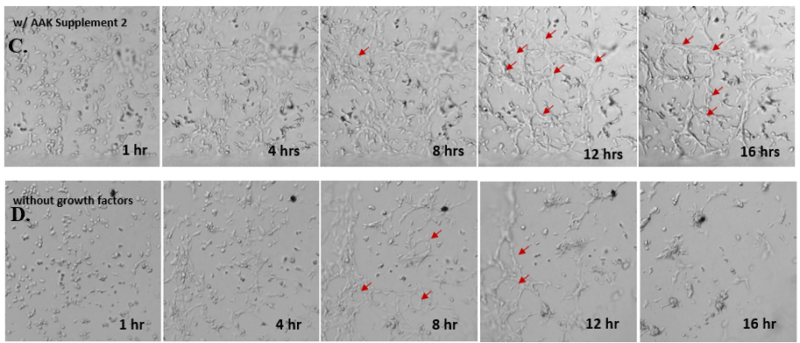
Figure 4. Comparison of the growth of endothelial cells on top of VitroGel AAK hydrogel with and without growth factor supplement. TOP LEFT) Time lapse video showing tube formation during 18 hours of HUVEC seeding on VitroGel AAK hydrogel with AAK Supplement 2 containing VEGFs; TOP RIGHT) Time-lapse showing HUVEC seeding without cell growth factors; C) HUVEC cell growth at various time points on top of the VitroGel AAK hydrogel with AAK supplement 2; D) HUVEC cell growth at various time points without cell growth factors. Red arrows show tube structure formation.
For the study on VitroGel AAK-HC hydrogel, we aimed to provide insight and address a novel aspect of angiogenesis concerning how mechanical properties of the matrix might affect tube formation of HUVECs. Therefore, we prepared the hydrogel with different concentrations to achieve a variety of mechanical strengths of the hydrogel matrix. The VitroGel AAK Supplement 2 was used to prepare all hydrogels to eliminate the effect of growth factors on cell growth in order to focus on the effect of the mechanical property. The results of these analyses are shown in Figure 5. Here, we show that stronger hydrogel mechanical strength (i.e., lower dilution ratios such as the 1:0) results in diminished angiogenesis, as the cells are more prone to aggregation resulting in cell death. When compared to softer hydrogel mixtures such as the 1:1 dilution or 1:3 dilutions, the 1:0 dilution ratio showed significantly less tube structure formation. In particular, after tube formation, cells on the surface of the 1:0 dilution hydrogel began to aggregate and form cell spheroids at roughly the 12-hour time point. A similar observation was seen at the 16-hour time point for the 1:1 dilution, while for the softest hydrogel (1:3 dilution), the cells were able to attach to the hydrogel surface and gradually form tube structures at 18 hours to a much greater degree. The ideal situation for a hydrogel should be that it assists cells in their ability to adapt to a particular substance and ultimately enable the cell to spread nicely and attach to the matrix to eventually induce tube formation. Overall, these data illustrate high mechanical strength is not optimal for tube structure formation and angiogenic properties. Likewise, these data illustrate that softer hydrogel concentrations provide better support for cell attachment and viability over a longer period.
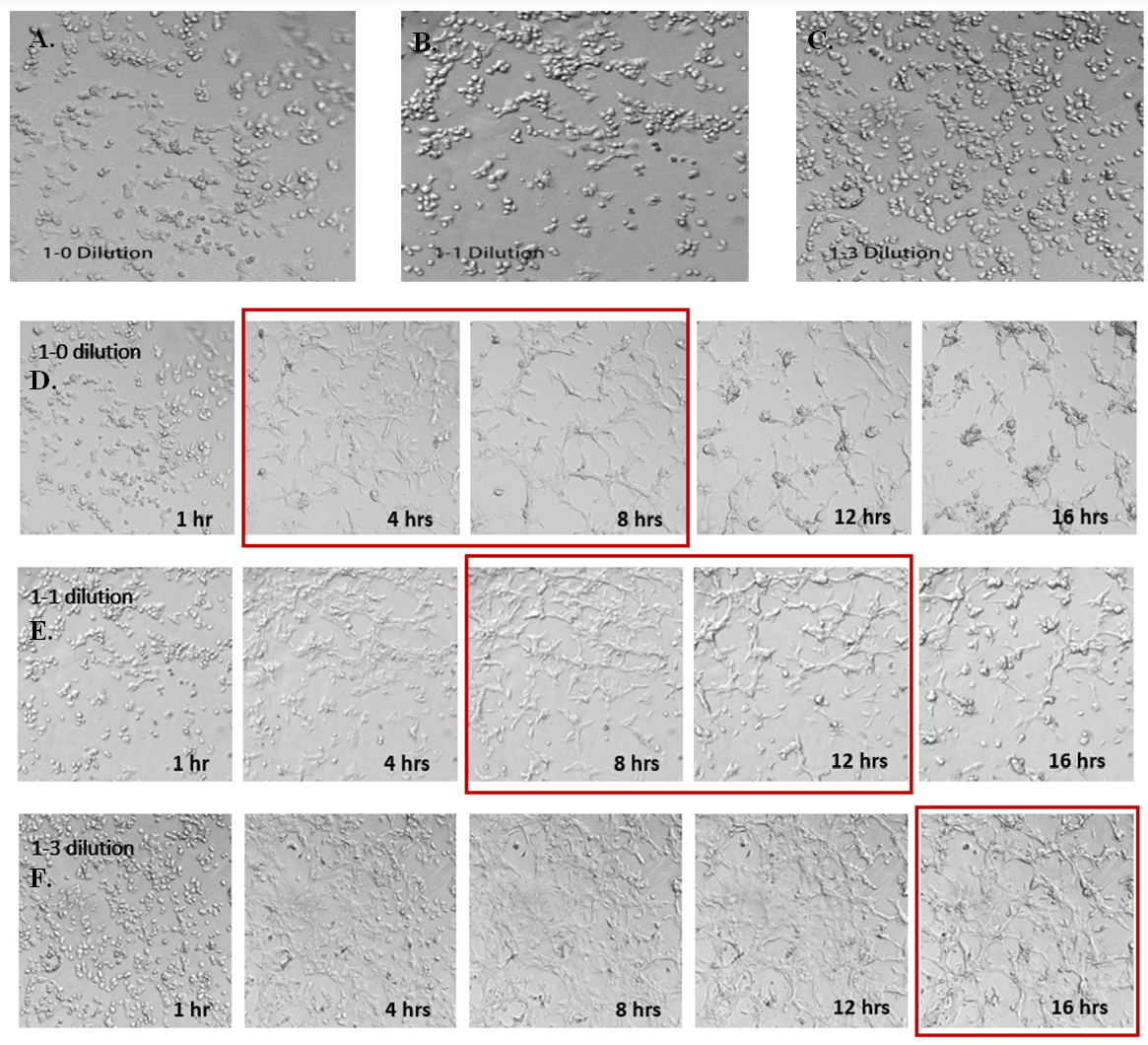
Figure 5. Effects of hydrogel mechanical strength on HUVEC tube formation. Different concentrations of VitroGel AAK-HC hydrogels were prepared by diluting the high concentration VitroGel AAK-HC with AAK-HC Dilution Solution at A, D) 1:0; B, E) 1:1; and C, F) 1:3 (v/v) ratios. The hydrogels were then mixed with AAK Supplement 2 (with VEGFs for tube formation) and incubated in the 96-well plate for 15 minutes. HUVECs cells were added on top of the hydrogel and observed under a live cell imaging system. The time-lapse videos show the tube formation in 18 hours after cells were added on top of hydrogel with different mechanical strengths. Static images illustrate various time points for each dilution. Red boxes indicate the strongest tube structure formation for each dilution.
Finally, we tested the 3D tube formation of HUVEC cells in both VitroGel AAK and VitroGel AAK-HC hydrogels. To demonstrate the 3D tube structure, we prepared z-stacked images of 3D tube structures formed within the hydrogels. As shown in Figure 6, we identified a clear 3D tube structure formed within the hydrogel using both the VitroGel AAK and VitroGel AAK-HC prepared with VitroGel AAK Supplement 2, suggesting that our xeno-free hydrogel system can support the 3D angiogenesis formation when encapsulated the HUVECs within the 3D hydrogel matrix (Figure 6). These results indicate significant advancement beyond the traditional 2D hydrogel coating methods with native ECM and show the ability to easy observation of 3D tube formation in response to modifications of matrix components themselves. This information is particularly important as it allows for more comprehensive investigations on complete cell-organoid interactions, which may provide further insight into angiogenic-related factors, including nutritional status, vascularization, and more.
A
B 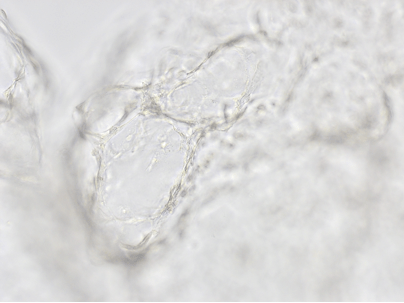
C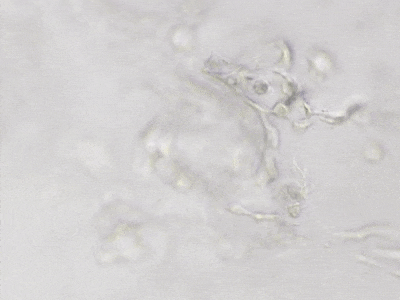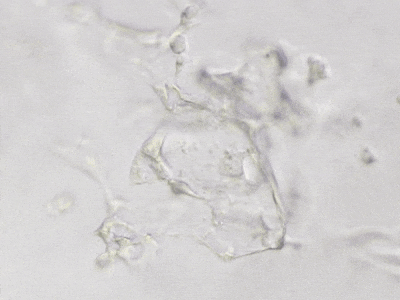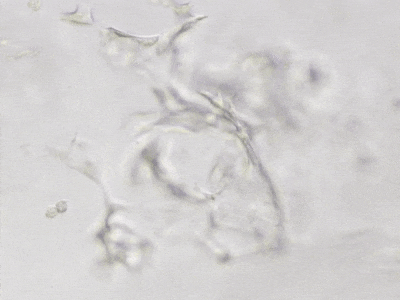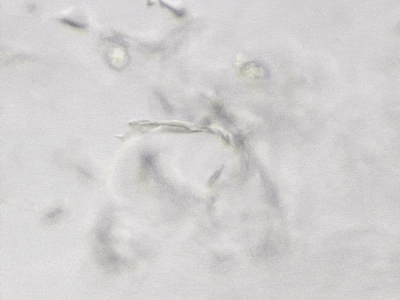
D
Figure 6. 3D tube structure of HUVEC cells cultured with the VitroGel AAK or AAK-HC. HUVEC cells were mixed with VitroGel AAK or AAK-HC hydrogel supplement with tube formation growth factors (AAK Supplement 2) and cultured for 7 days. A) Video showing z-stack images of the 3D tube structures formed within the VitroGel AAK hydrogel. B) Image showing tube networking structure within the VitroGel AAK hydrogel. C) Video showing z-stack images of the 3D tube structures formed within the VitroGel AAK-HC hydrogel. D) Image showing tube networking structure within the VitroGel AAK-HC hydrogel.
Concluding Remarks
The data described in this application note provide evidence for the utility of xeno-free VitroGel Angiogenesis Assay Kits as a research tool for both 2D hydrogel coating and 3D culture of human umbilical cord endothelial cells. This tool can be utilized in a variety of methods, some of which we have demonstrated here, where we exposed the HUVECs to different supplemental conditions or compared their morphology, proliferation, and viability with or without growth factors. Specifically, these data illustrate that growth factors such as VEGF in the hydrogel matrix considerably impact tube formation. Similarly, the hydrogel concentration itself is important for growth, proliferation, and tube formation as well, as hydrogels with higher mechanical strength result in drastically less (if any) tube formation, while softer hydrogels promote such growth. Altogether, these robust, powerful VitroGel Angiogenesis Assay Kits represent tunable tools that closely mimic the in vivo endothelial microenvironment, which encompasses cell-to-cell and cell-to-environment interactions that shape angiogenesis. We hope these tools enable researchers to study more comprehensive mechanisms underlying angiogenic processes and provide them with easy access to build tissue models for applications such as drug screening, invasion assays, and cancer research.
References
- Amann, A., Zwierzina, M., Koeck, S., Gamerith, G., Pechriggl, E., Huber, J. M., Lorenz, E., Kelm, J. M., Hilbe, W., Zwierzina, H., & Kern, J., (2017) Development of a 3D angiogenesis model to study tumour – endothelial cell interactions and the effects of anti-angiogenic drugs. Scientific Reports, 7(1).
- Jacobs, K. A., & Gavard, J., (2018) 3D Endothelial Cell Migration. Methods in Molecular Biology , 1749, 51–58.
- Stepanova, D., Byrne, H. M., Maini, P. K., & Alarcón, T. (2021). A multiscale model of complex endothelial cell dynamics in early angiogenesis. PLOS Computational Biology, 17(1), e1008055.
- Rajendran, P., Rengarajan, T., Thangavel, J., Nishigaki, Y., Sakthisekaran, D., Sethi, G., & Nishigaki, I. (2013). The Vascular Endothelium and Human Diseases. International Journal of Biological Sciences, 9(10), 1057–1069.
Links To Products Used
All product names, logos, brands, trademarks and registered trademarks are the property of their respective owners.