Advanced 3D Cell Models
Establish In Vitro Intestinal Model with Macrovascular Endothelium Using the VitroGel® Hydrogel System
Advanced 3D Cell Model
Introduction
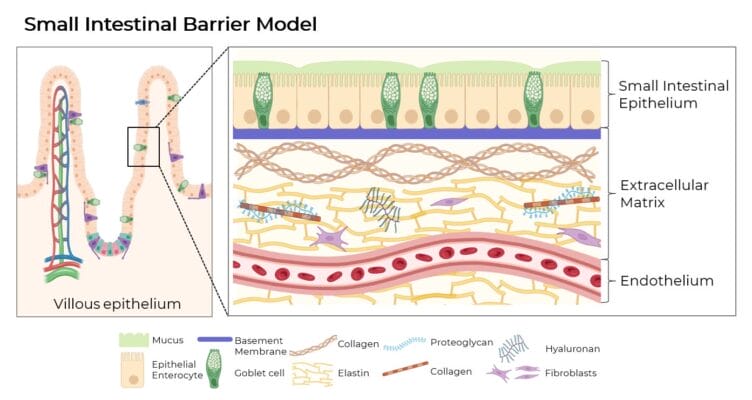
In vitro intestinal models serve as vital tools for studying biological processes, drug absorption and cytotoxicity while reducing the need for animal testing. Typically, these models consist of enterocytes and mucus-producing cells that are cultured for three weeks and closely mimic the human epithelium. Key components also include the extracellular matrix (ECM) for structural support and promotion of cellular processes, as well as a robust vascular network consisting of endothelial cells for transport and immune cell coordination.
The xeno-free bio-functional VitroGel® hydrogel system can emulate the native ECM appropriate for an in vitro model of the human small intestine. The hydrogels support the development of an artificially vascularized substrate, surrounded by the key aspects of the extracellular environment that have a concrete impact on the molecular transport processes.
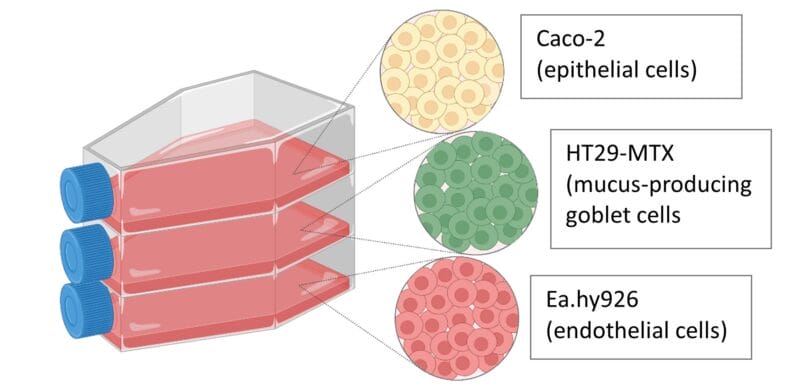
Here, we present a complete workflow developed by Dr. Eva Roblegg’s group from the University of Graz to establish the advanced in vitro vascularized intestinal model by cocultivation of Caco-2, HT29-MTX and EA.hy926 cells using the VitroGel hydrogel system.
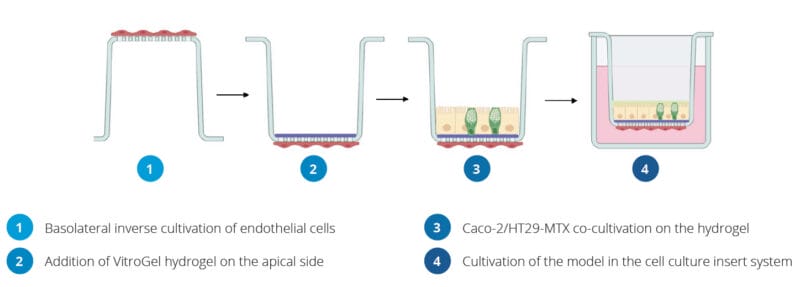
Workflow Of The Establishment Of The In Vitro Intestinal Model
Protocol
Culture and maintain Caco-2, HT29-MTX and EA.hy926 cells
Caco-2 cells, mucus-producing HT29-MTX cells and the endothelial cell line EA.hy926 were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% Fetal Bovine serum (FBS), 1% penicillin−streptomycin (Penstrep), and 1% Nonessential Amino Acid Solution (NEAA) at 37 °C in 5% CO2 with media changes every two to three days.
Establishing the In Vitro Co-culture Model
- Inverse Cultivation of Endothelial Cells on the Basolateral Side.
- Place an insert from the 24-well VitroPrime Cell Culture Inserts upside down in a 12-well plate.
- Add 1 × 104 cells/well EA.hy926 cells on the basolateral side of the filter (V=100 µl).
- Incubate the cells at 37 °C and 5% CO2 for 2 hours.
- Plate the insert back to the original 24-well plate in the default direction and add DMEM to the basolateral side of the insert (V=600 µl).
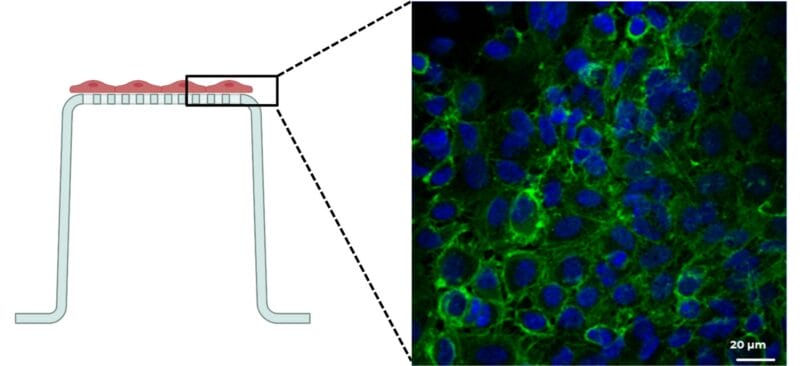
Image of successfully cultivated endothelial cells on the basolateral side of the insert. The figure illustrates a confluent endothelial cell layer on the basolateral side of an insert filter after 7 days of cultivation. Nuclei are shown in blue (λEx=405 nm); the cytoskeleton is shown in green (λEx=488 nm).
- Apical Cultivation of a Caco-2/HT29-MTX Coculture on VitroGel.
- Mix the VitroGel Hydrogel Matrix (Cat#: VHM01) with DMEM in a ratio of 4:1. Subsequently, add 40 μl of the VitroGel/DMEM mixture to the cell culture insert.
- Incubate the insert for 30 min at 37 °C.
- Prepare the mixture of Caco-2 and HT29-MTX cells at a 7:3 ratio with a total amount of 1.5 × 105 cells in 300 µl per well and add the Caco-2/HT29-MTX cell suspension onto the top of VitroGel.
- Culture the cells for 7, 14, and 21 days with medium change three times a week.
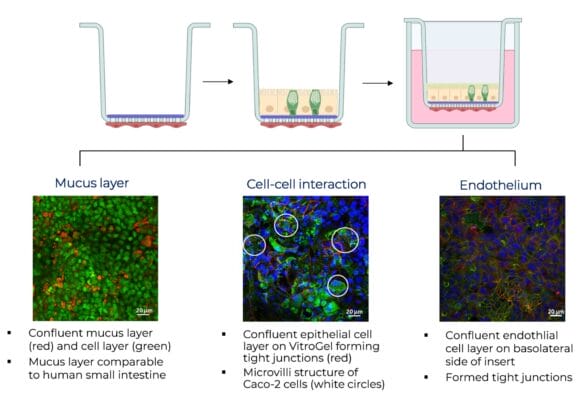 The first figure (A) shows a confluent mucus layer on the coculture of Caco-2 cells and HT29-MTX cells in a ratio of 7:3 cultivated on VitroGel for 14 days. The visualization was performed using Acridine Orange. Thereby, the red color (λEx=650 nm) indicates the mucus produced by the HT29-MTX goblet cells and the green color indicates the cells (λEx=525 nm).In (B), a confluent coculture comprising Caco-2 and HT29-MTX (7:3) cells cultivated on VitroGel is illustrated after 14 days of cultivation. (C) depicts the endothelial cell layer on the basolateral side of the filter at the same time point. In both figures, cell nuclei were stained in blue (λEx=405 nm), cytoskeleton in green (λEx=488 nm), and Occludin in red (λEx=543 nm). The white circles indicate the microvilli structure of Caco-2 cells.
The first figure (A) shows a confluent mucus layer on the coculture of Caco-2 cells and HT29-MTX cells in a ratio of 7:3 cultivated on VitroGel for 14 days. The visualization was performed using Acridine Orange. Thereby, the red color (λEx=650 nm) indicates the mucus produced by the HT29-MTX goblet cells and the green color indicates the cells (λEx=525 nm).In (B), a confluent coculture comprising Caco-2 and HT29-MTX (7:3) cells cultivated on VitroGel is illustrated after 14 days of cultivation. (C) depicts the endothelial cell layer on the basolateral side of the filter at the same time point. In both figures, cell nuclei were stained in blue (λEx=405 nm), cytoskeleton in green (λEx=488 nm), and Occludin in red (λEx=543 nm). The white circles indicate the microvilli structure of Caco-2 cells.
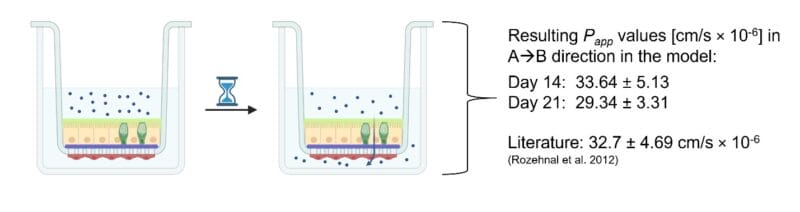
- Drug Transport Studies with the model drug Antipyrine.
- Establish the model following the instructions of Step 2.
- Prepare the Antipyrine solution at a concentration of 1.88 mg/ml in Krebs Ringer Bicarbonate Buffer (KRBB) and pre-warm the solution to 37 °C.
- Gently wash the cells with KRBB.
- To test apical to basolateral permeability (A→B), add 300 µl of the drug solution to the apical side of the insert (i.e., donor compartment) and 600 µl blank KRBB to the basolateral compartment (i.e., acceptor compartment) and incubate at 37 °C.
- Completely remove the KRBB from the acceptor compartment at predetermined time points (e.g., 15, 30, 45, 60, 90, 120, 150, and 180 min) and replace it with fresh, prewarmed KRBB.
- For quantification of the permeated drug through the model into the basolateral compartment, high-performance liquid chromatography (HPLC) can be performed.
- Based on the data obtained from the HPLC measurements, the apparent permeability coefficient (Papp, cm/s × 10-6) can be calculated using the following equation:
Papp = (dm/dt)/ (C0 × A)
dm/dt = amount of drug permeated into the acceptor compartment over time (s)
A = contact area of the insert
C0 = initial drug concentration in donor compartment
Read the Protocol
Establish In Vitro Intestinal Model with Macrovascular Endothelium Using the VitroGel® Hydrogel System
This protocol was contributed by Dr. Eva Roblegg’s group from the University of Graz and modified by TheWell Bioscience.
Acknowledgement

Dr. Eva Roblegg’s group from the University of Graz
Source: https://pharmazie.uni-graz.at/de/forschen/pharmazeutische-technologie-biopharmazie/drug-delivery-and-advanced-manufacturing/
References
- Zeiringer et al., “Development and Characterization of an In Vitro Intestinal Model Including Extracellular Matrix and Macrovascular Endothelium”, Mol. Pharmaceutics 2023
- Schimpel et al. “Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles”, Mol. Pharmaceutics 2014
- Volpe et al., “Classification of drug permeability with a Caco-2 cell monolayer assay”, Clin. Res. Regul. Aff. 2007
- Rozehnal et al. “Human small intestinal and colonic tissue mounted in the Ussing chamber as a tool for characterizing the intestinal absorption of drugs”, Eur. J. Pharm. Sci. 2012
Products Used