Research Highlights
Finding Living Needles in a 3D Haystack
Institutions:
National Institute of Standards and Technology (NIST) and National Institutes of Health (NIH), Maryland, USA
Team:
Babakhanova, G., Agrawal, A., Arora, D., Horenberg, A., Budhathoki, J.B., Dunkers, J.P., Chalfoun, J., Bajcsy, P., & Simon Jr., C.G.
Disease Model:
Tissue Repair
Hydrogel:
VitroGel® 3D
The use of 3D optical coherence tomography (OCT) proves to be a quick, easy, and non-invasive means to measure cell viability in 3D cell scaffolds that clinicians can use to repair damaged tissue.
The authors of this paper sought to find a reliable test to measure cell viability in a 3D scaffold, which is a complex that can be used in tissue engineering applications as a therapeutic composite for tissue recovery and repair. Live cells in such a scaffold are far more functional than non-living cells, but the determination of how well cells can live in an artificial matrix is not always straightforward, especially in a three-dimensional complex. This measurement would vastly improve the usefulness of 3D scaffolds in a clinical setting.
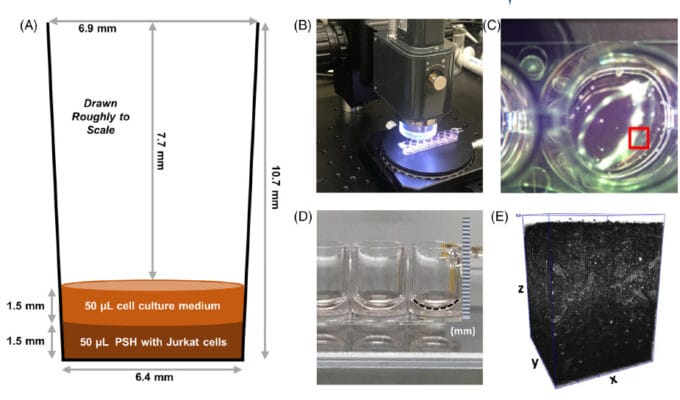
A team of bioengineers from NIST and the NIH employed 3D optical coherence tomography (OCT) to very quickly assess the how many, and where, live cells exist in a one cubic millimeter sample. The cells under consideration were human Jurket cells, which are immortal human T-lymphocytes that are useful in cell viability assays. Two types of these cells were prepared, live cells and heat-shocked dead cells. These cells were then encapsulated in TheWell Bioscience’s VitroGel ® 3D hydrogel matrix for further 3D cell culturing and analysis. Four experimental treatments were concocted, one with the VitroGel alone, one with live cells in the VitroGel, one with dead cells fixed in formaldehyde, and a final one with dead, heat-shocked cells.
The cells could be assayed for living state using two dyes visible to OCT: acridine orange (AO) and propidium iodide (PI). In addition, an ATP-based luciferase assay could infer living cells by the production of ATP through light production. Assays were performed by the authors on the various VitroGel encapsulated cells in 8-well stripwell plates by OCT imaging. By the use of the four experimental treatments, the investigators could easily construct microscopic means to subtract the background of the dead cells and compute metrics for living cells embedded in a 3D culture matrix. They obtained a very powerful (ca. 41X) signal-to-noise ratio in this regard. This result was repeatable over five repeat experiments. The team could determine the locations of the live cells within the matrix, and found that they were reasonably evenly distributed. The measurements took less than three minutes using 930 nm light.
The results of these experiments demonstrated that cell viability could be quickly determined in a 3D scaffold without the use of a troublesome label such as an MRI contrast agent, a fluorescent tag, or a radioactive tracer. The authors claim that their technique provides a new, powerful “large field of view” technique that can provide time-series information on cell viability in thick 3D scaffolds that are poised to be a valuable tool in wound healing clinical applications.


