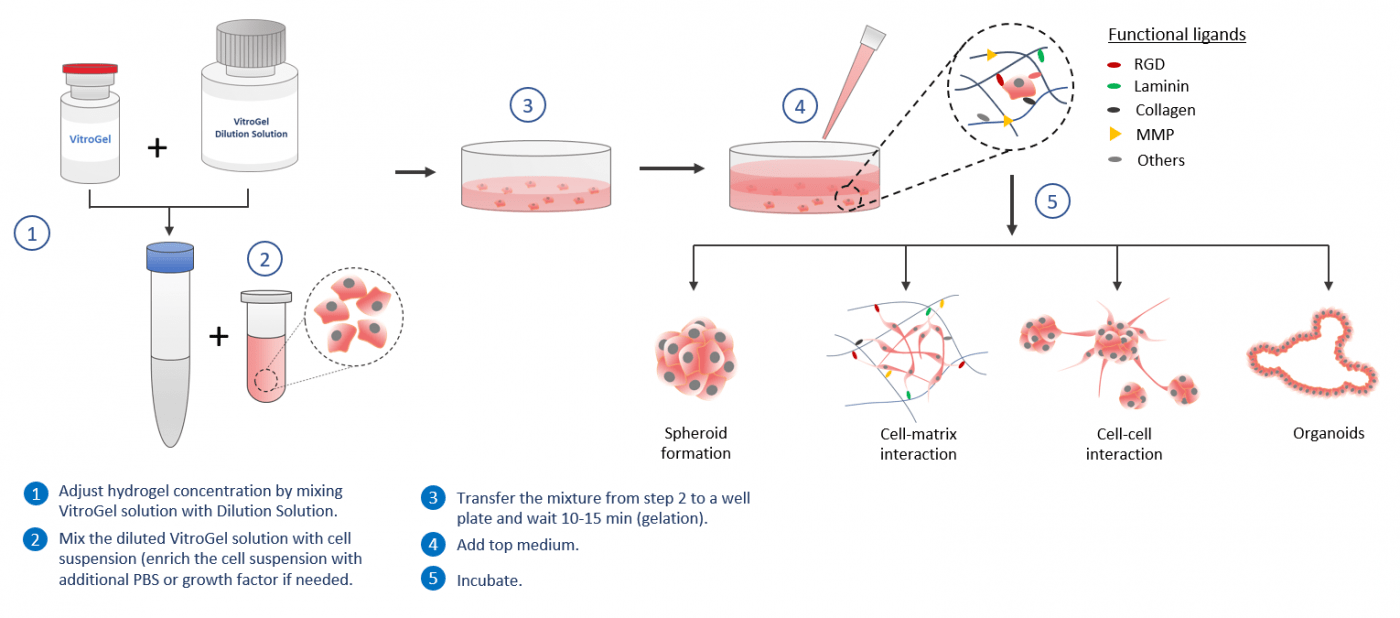
3D Cell Model
Using VitroGel® to build a 3D cell model is easy and versatile. By simply mixing the hydrogel solution with cell suspension at room temperature, the operation process can be done in 20 minutes (including a 10-15 min waiting time for hydrogel stabilization). The mechanical properties of the hydrogel can be tuned by adjusting the hydrogel concentration with VitroGel® Dilution Solution. Biological functional ligands (such as RGD, collagen, laminin, and MMP) are modified in different versions of VitroGel® to closely mimics the natural extracellular matrix (ECM) environment. The VitroGel® system can be “mixed and matched” with each other. Scientists can tailor create their 3D culture micro-environment by blending different VitroGel® systems for different applications. Drug screening, immunofluorescence analysis, and cytotoxicity assays can perform in hydrogel directly. Cells cultured in this system can be easily harvested out with VitroGel® Organoid Recovery Solution for downstream analysis or subculture.
Workflow
Data Examples
By using the VitroGel® to build in vitro 3D cell models, scientists can get full control to examine cellular responses in a more physiologically relevant context. The biomatrix microenvironment can be manipulated in several different ways: Mechanical Strength, Functional Ligands, Degradation, and Serum/Growth Factors/Cytokine/Chemokine
Mechanical Strength
Mechanical Strength
Cells cultured in 3D biomatrix are affected by the mechanical strength of the matrix structure. In VitroGel® , the hydrogel strength can adjust by a simple dilution process. The typical gel strength range from 10 to 4,000 pa and the customized higher concentration hydrogel can get over 20,000 Pa.
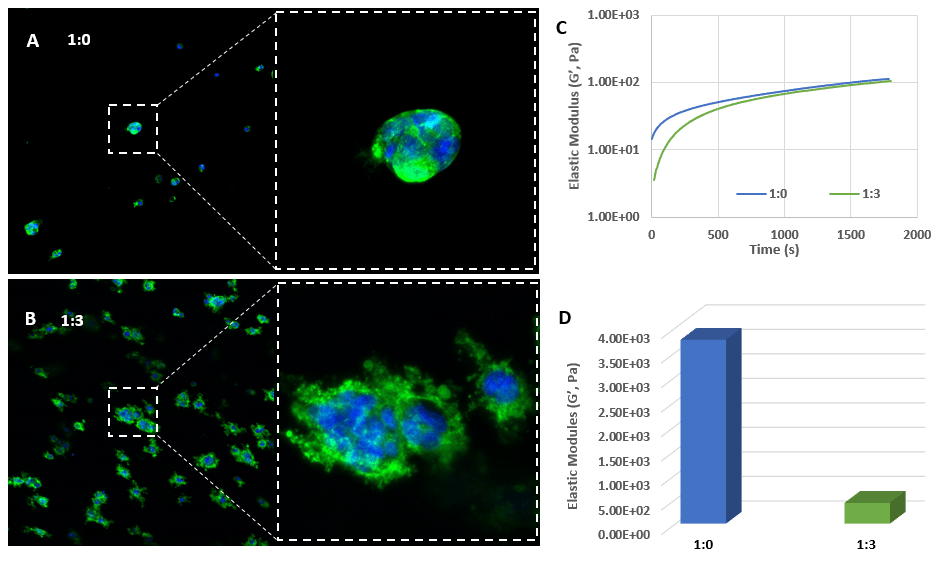
PANC-1 cells culture in VitroGel® system at different dilutions.
The images show the growth of PANC-1 cells that were encapsulated in 3D VitroGel® matrix for 7 days. At 1:0 dilution (A), the cells perform as a spheroid morphology with a lower proliferation rate than those cultured at 1:3 dilution (B). Looking an enlarged view of the ActinGreen / NucBlue staining, in Figures A and B, reveals increasing complexity and dispersion of the ECM generated by the cell-cell contact of PANC-1 cells when maintained in a softer hydrogel (1:3 dilution).
The time sweep elastic modulus data (C) indicate the hydrogel formation after mixing with cells to maintain the homogeneous 3D cell suspension. Image D shows the different final hydrogel strengths (D) at two dilutions (3,500 -4,000 Pa for 1:0 dilution and 100 – 300 Pa for 1:3 dilution), which lead to the different cell responses in this study.
Read application note to learn moreFunctional Ligands
Functional Ligands
Functional ligands perform important roles for in vitro cell culture. With VitroGel® , scientists can select hydrogel with different functional adhesive ligands, such as RGD, collagen, and Laminin. Since the functional versions of VitroGel® are based on the same matrix platform, scientists can combine and vary the concentration of individual functional ligands to form a heterogeneous customized microenvironment.
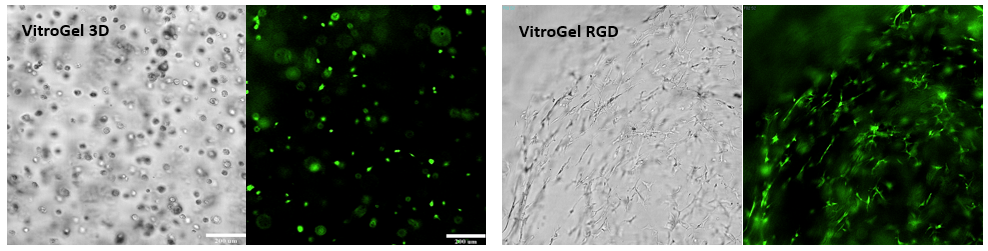
Bone marrow cells (OP9) 3D cultured in VItroGel® 3D and VitroGel® RGD.
The RGD-modified VitroGel promotes cell proliferation and cell-cell communication during the 3D cell culture. The strong cell-matrix interaction leads to the cell networking structure. While cultured in VitroGel 3D, the cells maintain the individual sphere structure without networking structure.
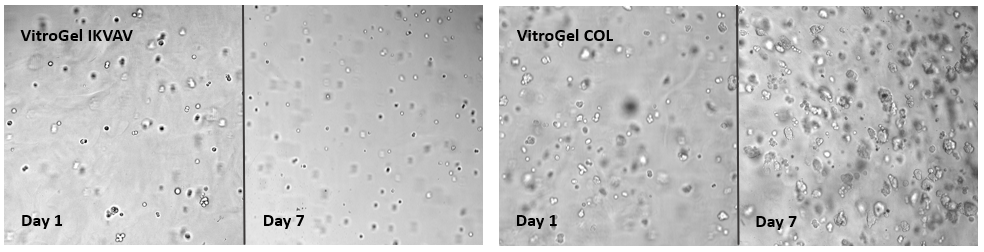
Breast cancer cells (MCF-7) cultured on VitroGel® IKVAV and VitroGel® COL.
Besides considering the hydrogel with or without the adhesive binding ligands (example above), choosing the right corresponding functional ligand is also very important. The images show the growth of MCF-7 from day 1 to day 7 on VitroGel® IKVAV and VitroGel® COL. The collagen functional ligand can support cell proliferation and sphere formation better than the IKAV ligand.
Degradation
Degradation
Metastatic cells degrade the local ECM to control invasion and dissemination into new tissue. The MMP-modified VitroGel® can be used for this study. The hydrogel can mix and match with other versions of VitroGel® to study the process of MMP degradation and cell-matrix interaction for cell mobility.
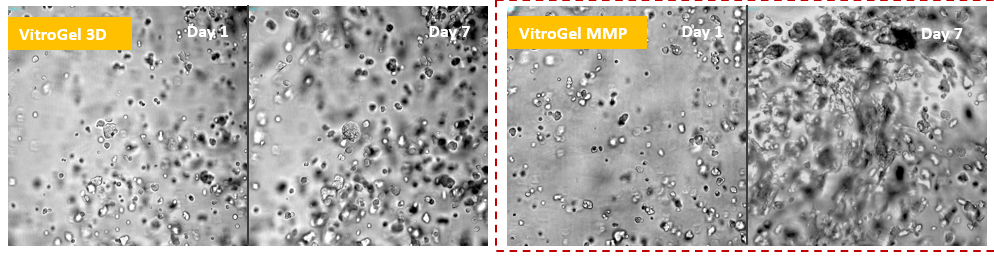
Glioblastoma cells (SNB 75) 3D cultured in VitroGel® 3D and VItroGel® MMP
Using the MMP-modified VitroGel® MMP, scientists can study cell mobility by degrading the local ECM during cell proliferation and invasion. The images show that the SNB-75 cells perform a strong ECM degrading capability in VitroGel® MMP.
VitroGel® MMP is commonly used for “mix and match” by blending with other functional VitroGel to create a microenvironment with both bio-functional ligands and degradability.
Serum | Growth Factors | CyTOKINE | CHEMOKINE
Serum/Growth Factors/Cytokine/Chemokine
VitroGel® is the xeno-free system. Scientists get full control of the microenvironment, including the serum and growth factors. These compounds can add to cell suspension before mixing with hydrogel to create an enriched hydrogel matrix or added after the hydrogel formation to feed cells.
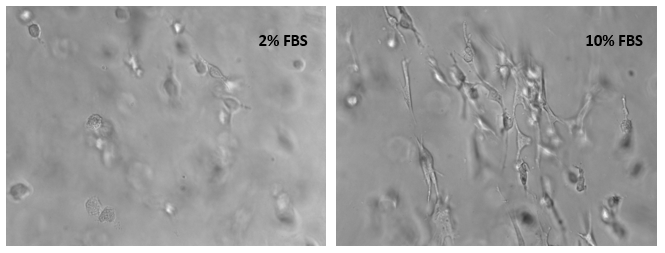
Bone marrow cells (OP9) 3D cultured in VitroGel® RGD with 2% and 10% FBS.
The xeno-free VitroGel® system allows scientists to get full control of the bioactive compositions of the biomatrix. The cell suspensions in this test were prepared in a culture medium with 10% and 50% FBS. After mixing the VitroGel® hydrogel solution with cell suspension at a 4:1 (v/v) ratio, the cells were encapsulated in a hydrogel matrix with 2% and 10% final FBS concentrations respectively. The images were taken 18 hours after cell seeding.