Application Notes
Functionalizing Xeno-Free VitroINK® Bioinks to Promote Cell-Cell Interactions Between Human-Induced Pluripotent Stem Cell-Derived Testis Cells for In Vitro Spermatogenesis
Application Note
Meghan Robinson, Ryan Flannigan
Vancouver Prostate Centre, University of British Columbia


Introduction
The differentiation of sperm, termed spermatogenesis, is coordinated by somatic cells within the seminiferous tubules of the testis niche. Spermatogonial stem cells – the progenitor cells which give rise to sperm – form physical attachments to somatic cells called Sertoli cells, which guide their differentiation through a program of paracrine signaling.1 Human in vitro models of spermatogenesis are needed for experimental tools and therapeutic platforms. As such, the ability to re-establish an in vitro microenvironment wherein such germ-somatic cell interactions take place is needed.
In this study, we explored xeno-free biofunctional VitroINK® bioinks (TheWell Bioscience) for their ability to promote cell-matrix and cell-cell interactions to aid in re-establishing the somatic cell niche. Each VitroINK was functionalized with biomimetic peptide motifs known to be present within the testis niche, namely YIGSR (laminin), COL (collagen), RGD (fibronectin), or MMP (matrix metalloproteinases). As controls, we compared these with two bioinks formulated with natural matrix proteins laminin 521 (LAMININK 521, CELLINK) or fibrin (TissuePrint, Axolotl Biosciences). Testicular cells were mixed into the bioinks and printed with a CELLINK BIO X6 extrusion bioprinter. Results identified the functionalized VitroINKs as highly supportive of testicular cell viability, cell-matrix, and cell-cell interactions, while natural bioinks promoted viability but overall lacked structural integrity and cell-cell or cell-matrix interactions. Moreover, the printability of the VitroINKs into thin microfibres on the scale of seminiferous tubules was excellent in terms of resolution and stability. These bio-functionalized VitroINKs represent a promising means to develop 3-D bioprinted human testicular tissues.
Materials and Methods
Differentiation of human testicular cells from induced pluripotent stem cells and expansion
Human induced pluripotent stem cells (hiPSCs, iPS11-10, Cell Applications) were differentiated into spermatogonial stem cells, and the somatic cells comprising the testicular niche (Sertoli cells, Leydig cells, and peritubular myoid cells) and expanded as previously described.2
3-D bioprinting
HiPSC-derived testicular cells were mixed and resuspended in Stempro34 Serum Free Medium (SFM) with 1X Insulin-Transferrin-Selenium (ITS) Supplement, 60×10-6 M putrescine, 30×10-9 M sodium selenite, 30 μg/mL pyruvic acid, 1 μL/mL DL-lactic acid, 2×10-3 M L-glutamine, 5 × 10−5 M 2-mercaptoethanol, 1X MEM vitamin solution, 10−4 M vitamin C, 10 μg/mL d-biotin, 30 ng/mL β-estradiol, 60 ng/mL progesterone, 10×10-6 M retinoic acid, 100 ng/mL recombinant Stem Cell Factor (SCF), 100 ng/mL Bone Morphogenic Protein 4 (BMP4), 20 ng/mL Epidermal Growth Factor (EGF), 10 ng/mL Leukemia Inhibitory Factor (LIF), 10 ng/mL Glial Cell-Derived Neurotophic Factor (GDNF), 10 ng/mL Platelet-Derived Growth Factor BB (PDGFBB), 5×10-6 M 22(R)-hydroxycholesterol, 100 ng/mL follicle stimulating hormone (FSH), 10 ng/mL luteinizing hormone (LH), and 1×10-6 testosterone. The final cell concentration was 51×106 cells/mL. The suspended cell mixture was then mixed sequentially into MMP, YIGSR, COL, RGD, and Lam521 bioinks using the same cell-mixer, VitroINK Mixing Kit (TheWell Bioscience), and cell suspension to ensure consistency across bioink groups, at a 3:1 (v/v) ratio for a final cell concentration of 17×106 cells/mL in each bioink. Cell-loaded bioinks were given 15 minutes to settle at room temperature before printing.
Fibrin bioink was mixed and printed separately, as it required additional prep and printing time. hiPSC-derived testicular cells were mixed directly into the bioink for a final concentration of 17×106 cells/mL.
Each bioink was printed using a CELLINK BIO X6 extrusion bioprinter into 5 mm diameter circles on µ-Dishes or µ-Slides with uncoated coverslip bottoms (Ibidi) using a 26-gauge stainless steel needle (Rame-Hart). Minimum pressures for extrusion were 15 kPa for the fibrin bioink, 30 kPa for the Lam521 bioink, and 110 kPa for YIGSR, RGD, COL, and MMP bioinks.
Immunofluorescence
Cells were fixed and stained for tubulin (TUBB3), actin (phalloidin), and DAPI as described previously,3 without the final step of optical clearing.
Live/Dead Assay
Viability was examined after 7 days in culture using the Cyto3D® Live-Dead Assay Kit (TheWell Bioscience). 10 µL of the Cyto3D solution was added to each well that contained <500 µL print with 500 µL cover medium. The cells were then incubated at a 34 °C incubator for 15-30 minutes before imaging with an Olympus FV3000 confocal microscope.
Confocal microscopy
Printed constructs were imaged using an Olympus FV3000 confocal microscope. Stacks were acquired and presented as either the Z-projection of the maximum intensities or as 3-D reconstructions.
Results
Printability into microfibers
To mimic the scale and shape of the native architecture of seminiferous tubules – long tubules ~200-400 µm in diameter -, the cells were printed into microfibers using a 26-gauge stainless steel needle with an inner diameter of 254 µm (Figure 1). The low viscosity of the natural fibrin bioink caused it to pool upon printing at a lower pressure (15 kPa) and higher speeds (5 mm/s), while the natural laminin bioink similarly required the lower pressure (30 kPa) and high speed to deposit microfibers (3 mm/s). In contrast, the functionalized VitroINKs were printed at the minimum speed (1 mm/s) and higher pressure (110 kPa) to achieve finer microfibers closer to scale with seminiferous tubules.
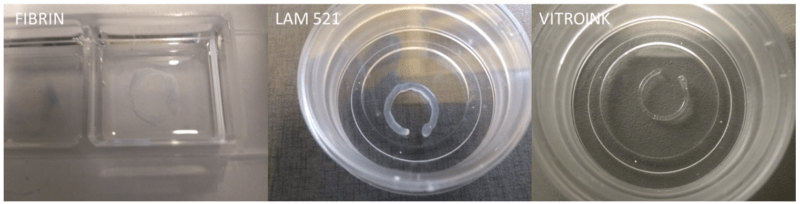
Figure 1: Printability of the bioinks into microfibers.
Due to the pooling behavior of the natural fibrin bioink, it was excluded from further analysis; however, it was noted that crosslinking with thrombin after printing transformed it into a mechanically strong and visco-elastic gel, suggesting that if crosslinking can be achieved during extrusion, it may be possible to create stable microfibers.
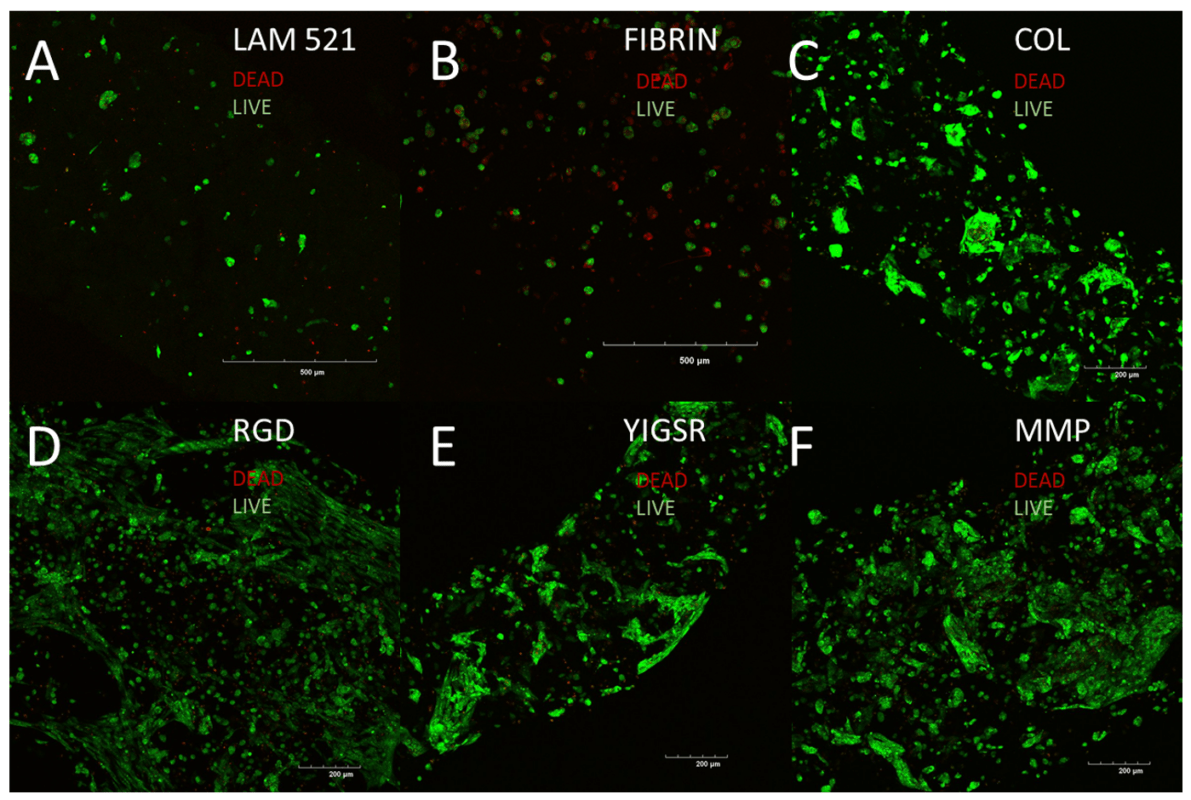
Viability
The viability of the printed cell-laden fibers was assessed after 7 days in culture. Live-Dead staining (Figure 2) showed similar levels of high viability among all bioinks. It also revealed notable differences in cell numbers between the VitroINK groups (Figure 2 C-F) and the Lam521 (Figure 2A) and fibrin-based (Figure 2B) groups, suggesting the VitroINKs encouraged mitotic activity.
Figure 2: Viability of the printed cells after 7 days of culture.
Viability
The viability of the printed cell-laden fibers was assessed after 7 days in culture. Live-Dead staining (Figure 2) showed similar levels of high viability among all bioinks. It also revealed notable differences in cell numbers between the VitroINK groups (Figure 2 C-F) and the Lam521 (Figure 2A) and fibrin-based (Figure 2B) groups, suggesting the VitroINKs encouraged mitotic activity.
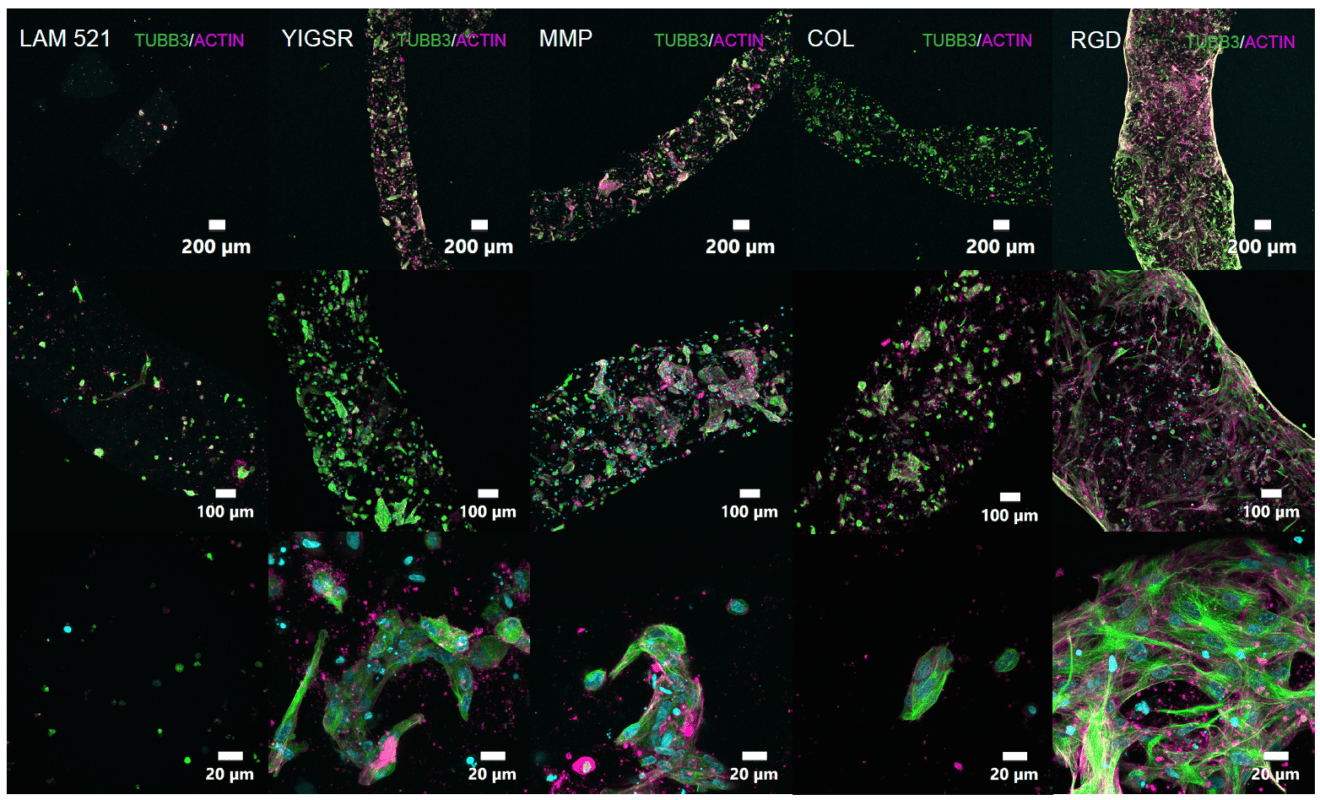
Figure 3: Cells were visualized with cytoskeletal stains Tubulin (TUBB3) and Actin after 7 days in culture.
Cell attachment and colonization were seen in all VitroINKs in stark contrast to the Lam521 bioink. Importantly, RGD was seen to promote migratory activity while MMP, YIGSR, and COL promoted colonization, illustrating specific cellular responses to different functional-ligand motifs.
Discussion
In this study, we explored the use of synthetic bio-functionalized VitroINKs to promote cell-matrix and cell-cell interactions amongst hiPSC-derived testicular cells as a step towards 3-D bioprinting human testicular tissue.
Our results illustrate that such functionalized VitroINKs can achieve excellent printability with the optimal biomaterial formulation and substantial improvements in cell growth by promoting the cell-matrix and cell-cell interactions. In contrast, conventional natural matrix bioink formulations of laminin 521 (CELLINK) and fibrin (Axolotl Biosciences) were difficult to print into stable constructs or appeared bioinert. An explanation may lie in the use of isolated cell attachment motifs as opposed to the complete native proteins. Besides numerous functional motifs, matrix proteins possess mechanical properties that can significantly affect the printability of a bioink, necessitating their dilution in bioink formulations.
Amongst the functional ligand modifications, RGD promoted the strongest cell-matrix interactions, generating fibroblast-like morphologies indicative of cell migration. In contrast, YIGSR, COL, and MMP promoted tight clustering of cells with defined boundaries reminiscent of colony formation. Together these findings indicate that in addition to promoting cell attachment, the behavior of the cells can be fine-tuned through the selection of appropriate functional motifs.
In summary, our findings show that VitroINKs formulated with functional matrix motifs support hiPSC-derived testicular cell attachment, migration, and colonization.
References
- Griswold, M. D. 50 years of spermatogenesis: Sertoli cells and their interactions with germ cells. Biol Reprod, 87-100, doi:10.1093/biolre/ioy027 (2018).
- Robinson, M., Witherspoon, L., Willerth, S. & Flannigan, R. A Novel Organoid Model of In vitro Spermatogenesis Using Human Induced Pluripotent Stem Cells. bioRxiv, 2021.2006.2004.447122, doi:10.1101/2021.06.04.447122 (2021).
- van Ineveld, R. L., Ariese, H. C. R., Wehrens, E. J., Dekkers, J. F. & Rios, A. C. Single-Cell Resolution Three-Dimensional Imaging of Intact Organoids. J Vis Exp, doi:10.3791/60709 (2020)
Links To Products Used
All product names, logos, brands, trademarks and registered trademarks are property of their respective owners.