Application Notes
Advanced 3D Luminal Breast Cancer Model in VitroGel® System: Long-term 3D Cell Culture and Co-culture with Fibroblast Cells
Application Note
Nana-Fatima Haruna, Bhavya Shah,John Huang
TheWell Bioscience, North Brunswick, NJ 08902
Introduction
Materials and Methods
Cell Maintenance
MCF-7 cells and NHDF cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 10% Fetal Bovine Serum (FBS), respectively. The cells were passaged when the cultures reached 80-90% confluence.
Long-term 3D Cell Culture
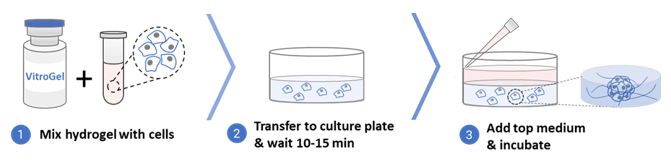
- Bring the VitroGel Hydrogel Matrix to room temperature or warm up to 37°C.
- Mix the hydrogel solution and the cell suspension at 2:1 (v/v) ratio.
- Add 50 µL of the hydrogel-cell mixture to each well of a 96 well plate.
- Wait 10-15 minutes at room temperature for hydrogel formation. Do not disturb the hydrogel by tilting or shaking the well plate during the formation process.
- Carefully add 50 µL cell culture medium (DMEM + 10% FBS) on top of the hydrogel.
- Place the well plate in an incubator and change the cover medium every other day.
* The volume of hydrogel and cover medium will depend on the sizes of the well plates. See protocol on the website for more information.
3D Co-culture
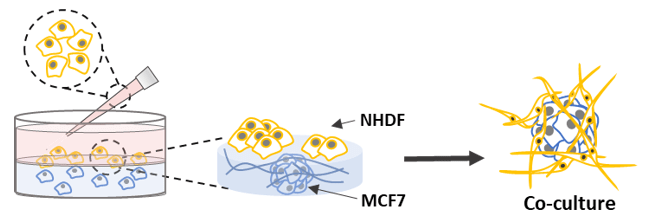
- Bring the VitroGel Hydrogel Matrix to room temperature or warm up to 37°C.
- Mix the hydrogel solution and MCF-7 cell suspension at 2:1 (v/v) ratio.
- Add 50 µL of the hydrogel-MCF-7 mixture to each well of a 96 well plate.
- Wait 10-15 minutes at room temperature for hydrogel formation. Do not disturb the hydrogel by tilting or shaking the well plate during the formation process.
- Carefully add 50 µL NHDF cell suspension on top of the hydrogel.
- Place the well plate in an incubator and change the cover medium every other day.
Proliferation Assay
The proliferation of the MCF-7 cells was measured using Cell Counting Kit-8 (CCK8) (Sigma-Aldrich) with the following steps.
Standard Curve:
- MCF-7 cell suspension was prepared in a complete DMEM medium with 30% FBS at a gradient of concentrations from 0.12 X 106 cells/mL to 1.2 X 106 cells/mL (5-7 gradient points in between).
- Prepare the 3D cell culture by mixing the hydrogel solution and MCF-7 cell suspension at 2:1 (v/v) ratio.
- Add 50 µL of the hydrogel-cell mixture to each well of a 96 well plate. The final cell concentrations in each wells were from 2,000 cells/well to 20,000 cells/well (5-7 gradient points in between, 3-5 repeats at each concentration point).
- Wait 10-15 minutes at room temperature for hydrogel formation. Do not disturb the hydrogel by tilting or shaking the well plate during the formation process.
- Carefully add 50 µL complete cell culture medium (with 10% FBS) on top of the hydrogel.
- Incubate cells at 37°C for 2 hours.
- Add 10 µL of CCK8 solution direct to the cover medium. Gently swirl the plate to make sure CCK-8 is evenly distributed.
- Incubate the plate in the dark (keeping away from light) at 37 °C for about 2 hours.
- Before reading the plate, swirl the plate gently to ensure homogeneous distribution of color.
- Measure the absorbance at 450 nm using a microplate reader.
- Make a standard curve using the cell numbers as the X-axis and the O.D. value as the Y-axis.
Measure Cell Proliferation:
- Prepare the 3D cell culture of MCF-7 in 96 well plate to measure the proliferation of the cells on days 0, 2, 4, 7, 11, and 14 (2,000 cells/well, 50 µL of the hydrogel-cell mixture and 50 µL of cover medium in each well, 3-5 repeat for each time point).
- At each time point, add 10 µL of CCK8 solution direct to the cover medium. Gently swirl the plate to make sure CCK-8 is evenly distributed. Note: Be careful not to introduce bubbles to the wells as they interfere with the O.D. reading. Optional: Remove 50 μL cover medium and add fresh 50 μL cover medium before adding the CCK-8 solution.
- Incubate the plate in the dark (keeping away from light) at 37 °C for about 2 hours.
- Before reading the plate, swirl the plate gently to ensure homogeneous distribution of color.
- Measure the absorbance at 450 nm using a microplate reader.
- Record the O.D. value and use the standard curve to convert the O.D. value to cell number
Viability Analysis
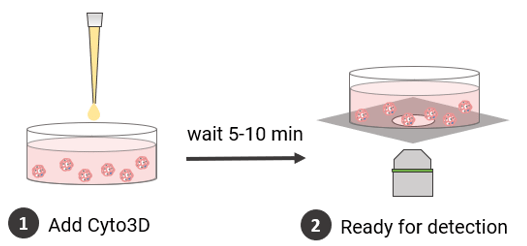
The viability of the long-term culture of the cells was monitored using Cyto3D™ Live-Dead Assay Kit (Cat. No. BM01, TheWell Bioscience, NJ).
- The Cyto3D Live-Dead Assay Kit was brought to room temperature.
- Add 2 µL of Cyto3D reagent to every 100 µL total volume in a well (50 µL hydrogel + 50 µL cover medium = total volume of 100 µL).
- Incubate the cells at 37°C for 5-10 minutes. The cells are ready for cell viability detection.
- The cells were imaged on a fluorescence microscope: live cells were observed using GFP filter while dead cells were observed using Texas red filter.
Fluorescence Imaging
- Remove the cover media from the top of the hydrogel.
- Wash the hydrogel with DPBS: add 100 µL DPBS to the top of the hydrogel and wait 1 minute before discarding. Wash 3 times.
- Add 100 µL of fixation solution (4% formaldehyde) and incubate at room temperature for 15-30 minutes.
- Remove the fixation solution and wash 3 times with 100 µL DPBS.
- Add 100 µL permeabilization solution (0.1% Triton X-100) and incubate at room temperature for 5 minutes.
Note: Permeabilization solution may cause the hydrogel to soften or slowly dissolve if incubated more than 15 min. Incubate at 4°C if more than 5 minutes - Remove the permeabilization solution and carefully wash 3 times with 100 µL DPBS.
- Add 100 µL blocking solution and incubate for 60 minutes at room temperature.
- Remove the blocking solution, add 100 µL F-actin staining solution*, and incubate in the dark for 30-60 minutes at room temperature.
- Remove the F-actin staining solution and wash 3 times with DPBS.
- Add nucleus staining solution* and incubate in the dark for 5 minutes at room temperature. After incubation, the sample is ready for fluorescent imaging.
*Prepare the working solution of F-actin staining solution and nucleus staining solution according to the product manual: add 2 drops of the reagent to 1 mL of DPBS.
Results and Discussion
MCF-7 cells were encapsulated in the hydrogel matrix for 3D cell culture. The cellular morphologies were monitored for 35 days. Figure 1 shows the 3D MCF-7 cell structures on days 1, 7, 14, and 35. Unlike the cell spheroid structure that is simply forming by aggregating cells into a round-shape colony in the U shape plate, handing drop plate or non-biologically functional hydrogel system, the functional VitroGel Hydrogel Matrix supports the formation of the luminal structure (red arrows in Figure 1) during cell spheroid formation since day 1. The luminal structure is an in vivo characterization of breast cancer spheroids, which plays a role in cancer progression. As the 3D spheroid structure grows, the luminal structures become more distinct at day 7 (Figure 1) when the cell clusters are about 50 µm in size.
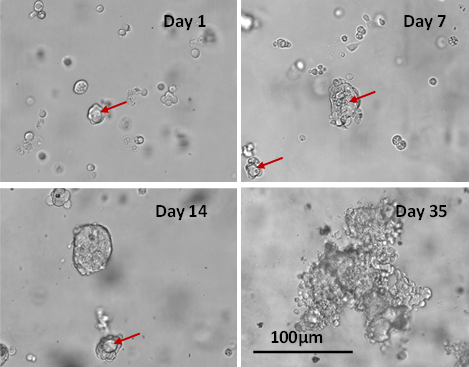
Figure 1. Long-term 3D culture of MCF7 breast cancer cells in VitroGel Hydrogel Matrix
The images show the morphology of MCF-7 cells at different stages of the long-term culture (day 1, 7, 14 and 35). The lumen structures (red arrow) were observed during cell spheroid formation from day 1. After day 7, some cells lost their polarity giving rise to the spheroid structure observed on day 14. The malignant stage of the spheroid continued to produce a heterogeneous mass of cells observed on day 35.
The fluorescence images in Figure 2 show organized nuclei formation and actin filaments surrounding the luminal structure. Interestingly, compared to the MCF-7 cells grown in Matrigel®, the 3D luminal structures can only show when cells were cultured in VitroGel Hydrogel Matrix. The interactions of the MCF7 cell clusters with VitroGel matrix produced a well-organized spheroid with cell-polarity consistent with the observation of in vivo tissue model. The 3D luminal breast cancer model in the VitroGel system provides an excellent model to study tumor growth, survival, and heterogeneity.
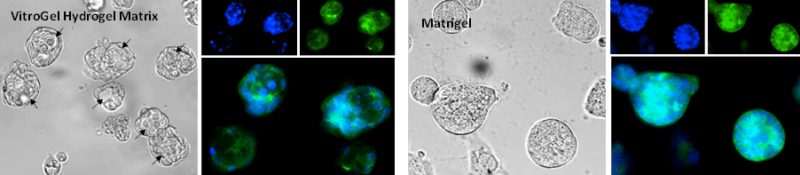
Figure 2. Human mammary breast cancer cells (MCF-7) cultured in VitroGel Hydrogel Matrix and Matrigel
3D luminal structures (arrows) were present when MCF-7 cells are cultured with VitroGel Hydrogel Matrigel. MCF-7 cells cultured with Matrigel only perform the spheroid structure. (Z-stack imaging system with 2D image projection was used. Blue: DAPI; Green: ActinGreen).
On day 14, the lumen-containing spheroids matured into a mass of cells with strong cell-cell bonds (Figure 1). The cell proliferation assay shows that the cell number increase from 2000 cells/well to about 132,000 cells/well in 14 days, which is more than 60-fold expansion (Figure 3). The cells kept growing after 14 days. The disorganized architecture and loss of lumen structures are because of the malignancy of the cell line. By day 35, the spheroid lost polarity and developed an apoptotic center (Figure 1) 1, 3, 6. The cell viability on day 35 was evaluated with Cyto3D Live-Dead Assay. Figure 4 shows the live-dead images of 3D MCF-7 cell spheroids: the sphere was viable as indicated by the green with a necrotic/ apoptotic zone in the core of the sphere. The cell organization is also scattered, indicating malignancies7. The results show the ability of VitroGel Hydrogel Matrix to support the long-term culture of 3D MCF-7 spheroid with excellent cell viability.
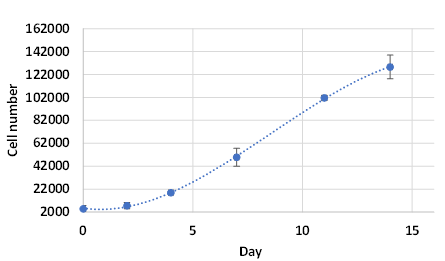
Figure 3. The proliferation of MCF-7 in VitroGel Hydrogel Matrix
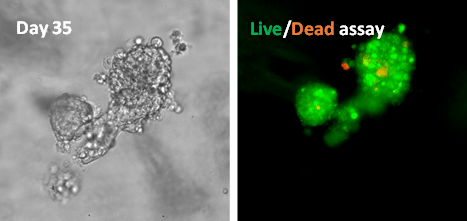
Figure 4. Live/dead assay of MSF7 cultured in VitroGel Hydrogel Matrix for 35 days
Image shows disorganized metastatic organization of cells within the tumor while the viability image shows a small zone of dead cell (red) at the center of the spheroid as well as surrounding the sphere.
We further established a 3D co-culture model by encapsulating the MCF-7 within the hydrogel matrix for the luminal spheroid formation and adding NHDF cells on top of the hydrogel to invade inside the hydrogel matrix for 3D migration toward the breast cancer spheroid. In our previous study (Application Note: 3D Skin Model of Human Dermal Fibroblast Cells in VitroGel System), we have demonstrated that the VitroGel Hydrogel Matrix can support the growth of NHDF cells in both 3D culture and 2D hydrogel coating culture. The NHDF cells can attach to the surface of the hydrogel and migrate into the hydrogel for a 3D cellular network structure. Figure 5 shows that both MCF-7 cells and NHDF cells grow on hydrogel on day 1. MCF-7 cells grew as spheroid structure in the hydrogel, and NHDF cells extend as cellular networking morphology. On Day 5, the two cell types then develop a large 3D coherent structure with an outer fibroblast layer and a core of tumor-like 3D breast cancer cells. The NHDF cells invaded the hydrogel matrix and grew surrounding the breast cancer tumor. The organization of the cells within the spheroids promotes heterogeneity which is a significant contributor to drug resistance 2,4.
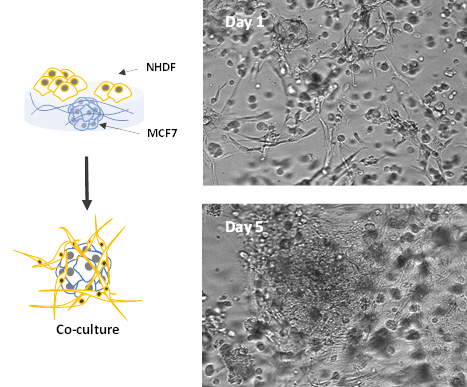
Figure 5. Co-culture of MCF-7 spheroids and NHDF cells in VitroGel Hydrogel Matrix
The images show the development of large 3D coherent structure with an outer fibroblast layer and a core of tumor-like 3D breast cancer cells.
This study shows that VitroGel Hydrogel Matrix supports the long-term cell growth and co-culture of tumor models. The phenotypic morphological features of breast cancer were observed during the development of the spheroids. Studying models with such complex and defined cell populations will produce more realistic, clinically, and physiologically relevant data in understanding the pathophysiology of the disease 9-11. Furthermore, the advanced 3D luminal breast cancer co-culture model in the VitroGel system provides insight into the relationship between cancer pathology and other cellular systems 10, 12. The results demonstrate the capability of the ready-to-use, functional xeno-free VitroGel Hydrogel Matrix to support the comprehensive 3D cell models of multiple cell types. The co-culture methodology allows researchers to explore other comprehensive 3D co-culture systems with the capability to monitor the changes in tumor evolution over time.
Reference
- Krause, S., Maffini, M. V., Soto, A. M. & Sonnenschein, C. The microenvironment determines the breast cancer cells’ phenotype: organization of MCF7 cells in 3D cultures. BMC Cancer 10, (2010).
- Comşa, Ş., Cîmpean, A. M. & Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Research 35, 3147–3154 (2015).
- Nguyen, T. & Shively, J. E. Induction of Lumen Formation in a Three-dimensional Model of Mammary Morphogenesis by Transcriptional Regulator ID4: ROLE OF CaMK2D IN THE EPIGENETIC REGULATION OF ID4 GENE EXPRESSION. The Journal of Biological Chemistry 291, 16766–16776 (2016).
- Amaral, J. B., Urabayashi, M. S. & Machado-Santelli, G. M. Cell death and lumen formation in spheroids of MCF-7 cells. Cell Biology International 34, 267–274 (2010).
- Vantangoli, M. M., Madnick, S. J., Huse, S. M., Weston, P. & Boekelheide, K. MCF-7 Human Breast Cancer Cells Form Differentiated Microtissues in Scaffold-Free Hydrogels. PLOS ONE 10, e0135426 (2015).
- Patil, P. U., D’Ambrosio, J., Inge, L. J., Mason, R. W. & Rajasekaran, A. K. Carcinoma cells induce lumen filling and EMT in epithelial cells through soluble E-cadherin-mediated activation of EGFR. Journal of Cell Science 128, 4366–4379 (2015).
- Stock, K. et al. Capturing tumor complexity in vitro: Comparative analysis of 2D and 3D tumor models for drug discovery. Scientific Reports 6, (2016).
- Wu, M. & Swartz, M. A. Modeling Tumor Microenvironments In Vitro. Journal of Biomechanical Engineering 136, 0210111–0210117 (2014).
- Yuan, H., Xing, K. & Hsu, H.-Y. Trinity of Three-Dimensional (3D) Scaffold, Vibration, and 3D Printing on Cell Culture Application: A Systematic Review and Indicating Future Direction. Bioengineering 5, 57 (2018).
- Nagai, Y., Yokoi, H., Kaihara, K. & Naruse, K. The mechanical stimulation of cells in 3D culture within a self-assembling peptide hydrogel. Biomaterials 33, 1044–1051 (2012).
- Dagogo-Jack, I. & Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nature Reviews Clinical Oncology 15, 81–94 (2017).
- Zoetemelk, M., Rausch, M., Colin, D. J., Dormond, O. & Nowak-Sliwinska, P. Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Scientific Reports 9, (2019).