Research Highlights
Taking Away a Notch in the Belt of Pancreatic Cancer
Institutions:
Taipei Medical University, National Cheng Kung University, National Sun Yat-Sen University, and the National Institute of Cancer Research, Taiwan, and the Mayo Clinic.
Team:
Tsai, Y.-C., Cheng, K.H., Jiang, S.S., Hawse, J.R., Chuang, S.E., Chen, S.L., Huang, T.-S., and Ch’ang, H.-J.
Disease Model:
Pancreatic Cancer
Hydrogel:
VitroGel® Hydrogel Matrix
A mouse model for pancreatic cancer is developed that demonstrates that the Krüppel-like factor 10 DNA-binding protein can suppress the Notch pathways, thereby dampening the likelihood of tumorigenesis.
There is a cascade of events that lead to most cancers, and pancreatic cancer is no exception. A particularly deadly form of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC), which rapidly metastasizes into tissues far from the site of origin. Finding both diagnostic and therapeutic tools for this form of cancer are desperately needed. We do know that a sequence of events, leading from altered DNA-binding to avoidance of natural tumor-forming inhibitors, is likely to trigger PDAC. But a clearer understanding of this pathway to destruction is needed to develop treatment plans in the clinic.
The authors of this study, a team of oncologists from Taiwan and the Mayo Clinic, focused on a particular transcription factor (i.e., a DNA-binding protein) called Krüppel-like factor 10 (KLF10). They knew that when expression of this protein was repressed, patients were more likely to develop PCAC. Thus, testing the various events that can occur when this protein is missing, in a mouse model, could provide valuable information.
A main goal, then, of the current study was to inject mice with pancreatic cancer cell lines (PANC-1 and MiaPaCa) that had been altered to vary their levels of KLF10. Then both genotypic and phenotypic studies of the cancer progression in these mice could be performed. This approach, xenografting, is a powerful way to use animal models to predict what could happen in humans.
To perform the xenograft, the team carefully injected genetically modified pancreatic cancer cells into the spleens of immunodeficient mice. In doing so, the scientists first suspended the cells in PBS in VitroGel® Hydrogel Matrix, which provides a robust xeno-free injection-optimized milieu that helps ensure that the xenografting takes place as planned. The mice were treated with various chemotherapeutic agents for three weeks, prior to being sacrificed and assayed for tumor growth and other important metrics.
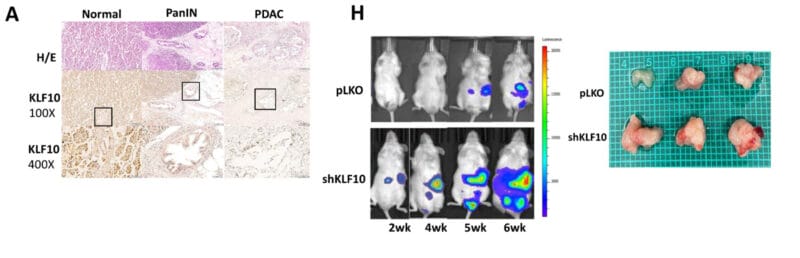
The researchers used immunochemical staining, fluorescence microscopy, microarrays, and PCR to follow tumor progression and response to putative treatments. They found that mice with KLF10 deficiencies tended to show faster tumor growth. Moreover, there were key other genetic changes that were significantly associated with tumorigenesis, including the lack of KLF10-promoted suppression of other (Notch) genes in those mice that developed larger tumors. If you work through all the negatives here, it turns out that KLF10 is a positive.
In general, this study demonstrated two key findings. First, that a potent mouse model for pancreatic cancer could be constructed fairly easily using xenografting. And second, that KLF10 clearly is involved in protecting cells from entering and progressing down the road to PDAC because the protein keeps the Notch overexpression at bay. This pair of important conclusions will certainly be valuable in making strides toward the clinical treatment of human pancreatic cancers in the future.