Application Notes
Using the Xeno-free VitroGel® Cell Invasion Assay Kits to Perform both Traditional and Complex Cell Mobility Studies
Application Note
Alejandra Ferrer Díaz, John Huang
TheWell Bioscience Inc., North Brunswick, NJ

Introduction
Cell invasion is a dynamic process that is critical during embryonic development, immunosurveillance, and wound healing. Cell invasion is an orchestrated mechanism that occurs due to cell attachment to the extracellular matrix (ECM) followed by proteolytic degradation of the ECM, resulting in movement towards the newly invaded site. Cell invasion is not only crucial for physiological processes but also for cancer cells to metastasize into local and distant regions within the body.
In vitro invasion assays have been developed throughout the years to better understand the processes underlying cell invasion. An extensively performed method is the traditional invasion assay, which requires the use of the Boyden chamber. The chamber comprises an insert that is first coated with hydrogel matrices and then placed inside cell culture well plates. The insert contains a porous membrane, creating a physical barrier between the upper compartment and the outer wall. The premise of this assay is that invasive cells degrade the hydrogel matrices in response to chemoattractants or other cell types placed in the outer well.
A significant challenge with the traditional invasion assay is the use of animal-based extracellular matrices (ECM): the components of animal-based ECM are not characterized and, as a result, their impact on cell invasion is unknown; the batch-to-batch variability of animal-based ECM can influence experimental findings and affect potential clinical applications; and the temperature-sensitive operation protocols make the homogeneous plate coating process time-consuming and difficult to adapt for automated liquid handlers for high-throughput assays. These challenges can be circumvented using VitroGel® hydrogels, synthetic xeno-free, bio-functional hydrogels resembling the physiological ECM with tunable biophysical and biochemical properties. Unlike the traditional animal-based ECM, VitroGel hydrogels can be adapted to evaluate how different mechanical strengths and functional ligands of hydrogel matrices, as well as chemokines, growth factors, cytokines, and serum within the hydrogel matrices or in the outer well affect cell mobility. This system offers a unique property that consists of embedding chemoattractants and chemical agents into the matrix to evaluate chemotaxis more closely. The VitroGel hydrogels are easy to use at room temperature, shortening the operation time from hours to less than 30 minutes and supporting high-throughput operation. VitroGel has been successfully used as a medium for cell invasion assays involving breast cancer cells [1], prostate cancer cells [2], and melanoma cells [3], among others. This powerful system is excellent for studying cell invasion and motility.
Here, we assessed the invasiveness of the highly aggressive glioblastoma tumor cells, U87-MG, by using various VitroGel-Based Cell Invasion Assay Kits. Both the “Ready-To-Use” VitroGel Cell Invasion Assay Kit (a versatile invasion kit that includes the ready-to-use VitroGel® Hydrogel Matrix and VitroPrime™ cell culture inserts) and various tunable VitroGel “High-Concentration” Cell Invasion Assay Kits (powerful versatile kits that include different types of tunable high-concentration VitroGel hydrogels and VitroPrime™ cell culture inserts) were used in this study. Besides the traditional chemoattraction assay, we evaluated how different hydrogel mechanical strengths and functional ligands (using the High-Concentration hydrogel versions) influence glioblastoma cell invasion towards a chemical gradient, which cannot be studied in an animal-based ECM system. Furthermore, we describe a novel and unique invasion assay that can be innovatively created using the VitroGel-Based Invasion Assay Kits, which consist of embedding chemoattractants and pharmacological agents into the matrix to evaluate chemotaxis.
Materials and Methods
Cell Culture
U87-MG glioblastoma cells were cultured in Minimum Essential Medium (MEM 1X) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 1% glutamine. Cells were passaged once 80-90% confluence was reached.
Cell Invasion Assay Kits
- Ready-To-Use Cell Invasion Assay Kit
- VitroGel Cell Invasion Kit (Cat# IA-VHM01-1P)
- Tunable High Concentration Cell Invasion Assay Kits
- VitroGel 3D Cell Invasion Assay Kit (Cat# IA-HC001-1P)
- VitroGel RGD Cell Invasion Assay Kit (Cat# IA-HC003-1P)
- VitroGel MMP Cell Invasion Assay Kit (Cat# IA-HC010-1P)
Additional Materials for Cell Invasion
- 1X phosphate saline buffer (1X PBS)
- 4% formaldehyde
- Trypan blue stain
- Cotton swabs
- Forceps
- Methanol
- Crystal violet stain
- Micropipette & low retention pipette tips
- Zeiss microscope
- Image J software
Methods
VitroGel® Cell Invasion Assay Kit (Ready-To-Use)
- Allow VitroGel Hydrogel Matrix and culture medium to reach room temperature.
- Add 1 mL VitroGel Hydrogel Matrix solution to 500 µL basal cell culture medium and gently pipette 5-10 times to homogenize the mixture. (Keep VitroGel Hydrogel Matrix solution and basal cell culture medium at 2:1 v/v mixing ratio).*If using cell culture medium with low salt concentration, such as RMPI 1640 medium, consider using 1:1 v/v mixing ratio. (Example, 500 µL VitroGel hydrogel solution to 500 µL cell culture medium). Note: If you need to add supplements such as cytokines, growth factors, chemokines, or chemical agents to the hydrogel matrix, add the 3X desired concentrations of supplement to the cell culture medium. The cell culture medium can then be mixed with the VitroGel hydrogel solution to get 1X final supplement concentration in the hydrogel matrix.
(Example: Prepare medium with 30 ng/mL of the cytokine. Mix VitroGel Hydrogel Matrix solution with the medium at a 2:1 v/v mixing ratio to obtain a final concentration of 10 ng/mL cytokine inside the hydrogel matrix). - Add 100 µL of the hydrogel mixture to each insert and ensure there is an even distribution of hydrogel covering the surface of each insert.
Note: If the experiment requires a thinner gel to evaluate invasion, adjust hydrogel volume to 50-100 µL per insert. - Allow the hydrogel mixture to solidify for 20 minutes at room temperature before adding the cells on top of the hydrogel.
- Prepare cell suspension in the desired culture medium (i.e., serum-free medium) at the concentration of 1-3 x 105 cells/ mL and add 100 µL of cell suspension on top of the hydrogel.
Optional Seeding Method: To ensure cells are seeded on the surface of the hydrogel, add 50% of the medium (without cells) on top of the hydrogel first. Wait 5-10 min, then add the remaining 50% of the medium with cells on top of the hydrogel. (Example: Add 50 µL medium (without cells) first; wait 10-5 min; then add 50 µl medium with 2-6 x 106 cells/mL on top). - Prepare cell culture medium with factors of interest (i.e., chemokines, cytokines, or serum), and add 500 µL of cell culture medium to the outer wells.
- Incubate cells in a humidified cell culture incubator at 37°C.
VitroGel® High-Concentration Cell Invasion Assay Kits (Tunable)
The protocol below is suitable for all VitroGel High-Concentration Cell Invasion Assay Kit versions. VitroGel RGD Cell Invasion Assay Kit is used as an example below. Replace the VitroGel RGD Cell Invasion Assay Kit with another high-concentration cell invasion assay kit version.
- Allow VitroGel® RGD hydrogel and basal medium to reach room temperature.
- Dilute VitroGel® RGD hydrogel solution with VitroGel® Dilution Solution for desired concentrations.
Refer to Table 1 for recommended volumes for different ratios.
Dilution Ratio (VitroGel/Dilution solution)
VitroGel® Dilution Solution Basal medium 1:0 1 mL 0 µL 250 µL 1:1 500 µL 500 µL 250 µL 1:2 300 µL 600 µL 225 µL 1:3 250 µL 750 µL 250 µL 1:4 200 µL 800 µL 250 µL 1:5 200 µL 1 mL 300 µL Table 1: Volume of VitroGel hydrogel solution, dilution solution, and cell medium for different dilution ratios for high-concentration hydrogels.
- Further, mix the diluted hydrogel solution with basal medium at a 4:1 ratio. Refer to Table 1 for recommended volumes for mixing.Note: If you need to add supplements such as cytokines, growth factors, chemokines, or chemical agents to the hydrogel matrix, add the 5X desired concentrations of supplement to the cell culture medium. The cell culture medium can then be mixed with the diluted VitroGel hydrogel solution at a 4:1 ratio to get 1X final supplement concentration in the hydrogel matrix.(Example: Prepare medium with 50 ng/mL of the cytokine. Mix diluted VitroGel hydrogel solution with the medium at a 4:1 v/v mixing ratio to obtain a final concentration of 10 ng/mL cytokine inside the hydrogel matrix).
- Add 100 µL of the hydrogel mixture to each insert and ensure there is an even hydrogel covering on the surface of each insert.
Note: If the experiment requires a thinner gel to evaluate invasion, adjust hydrogel volume to 50-100 µL per insert. - Allow hydrogel mixture to solidify for 20 minutes at room temperature before adding the cells on top of the hydrogel.
- Prepare cell suspension in desired culture medium (i.e., serum-free medium) at the concentration of 1-3 x 105 cells/mL and add 100 µL of cell suspension on top of the hydrogel.
Optional Seeding Method: To ensure cells are seeded on the surface of the hydrogel, add 50% of the medium (without cells) on top of the hydrogel first. Wait 5-10 min then add the remaining 50% of the medium with cells on top of the hydrogel. (For example, add 50 µL medium (without cells) first; wait 10-5 min; then add 50 µl medium with 2-6 x 106 cells/mL on top). - Prepare cell culture medium with factors of interest (i.e., chemokines, cytokines, or serum), and add 500 μL of cell culture medium to the outer wells.
- Incubate cells in a humidified cell culture incubator at 37°C.
Read the full protocol here: https://www.thewellbio.com/product/vitrogel-cell-invasion-assay-kit/
Results
VitroGel® Cell Invasion Assay Kit (Ready-To-Use)
Invasion of U87-MG glioblastoma cells towards a serum gradient
- Ready-To-Use Hydrogel – Cell Invasion Assay Kit:
VitroGel® Cell Invasion Assay Kit (CAT # IA-VHM01-1P) - Insert: VitroGel® Hydrogel Matrix mixed with MEM basal medium at 2:1 v/v mixing ratio
- Outer well: MEM basal medium (without serum) or MEM with 20% FBS
- Cells: U87-MG cells (3.8 x 104 cells per insert)
- Cell incubation time: 48 hrs
To perform the traditional invasion assay, VitroGel Hydrogel Matrix was mixed with MEM basal medium at 2:1 v/v ratio. The hydrogel mixture (100 µL) was added to each insert, followed by a 20-minute incubation at room temperature for hydrogel solidification. U87-MG cells (3.8 x 104 cells per insert) were then resuspended in MEM basal medium and placed on top of the coated inserts. The outer wells were replenished with MEM basal medium or MEM medium supplemented with 20% FBS (500 µL per well, Figure 1A). The cultures were incubated for 48 hours at 37°C. Following the incubation of the cells, we performed crystal violet staining to visualize cell invasion (Figures 1 B-C).
As expected, U87-MG cells exposed to medium supplemented with 20% FBS significantly invaded the hydrogel matrix compared to cells cultured in medium without serum (Figures 1B-C). These findings indicate that VitroGel Cell Invasion Assay Kit is a viable tool that can be used to examine cell invasion caused by a serum gradient.
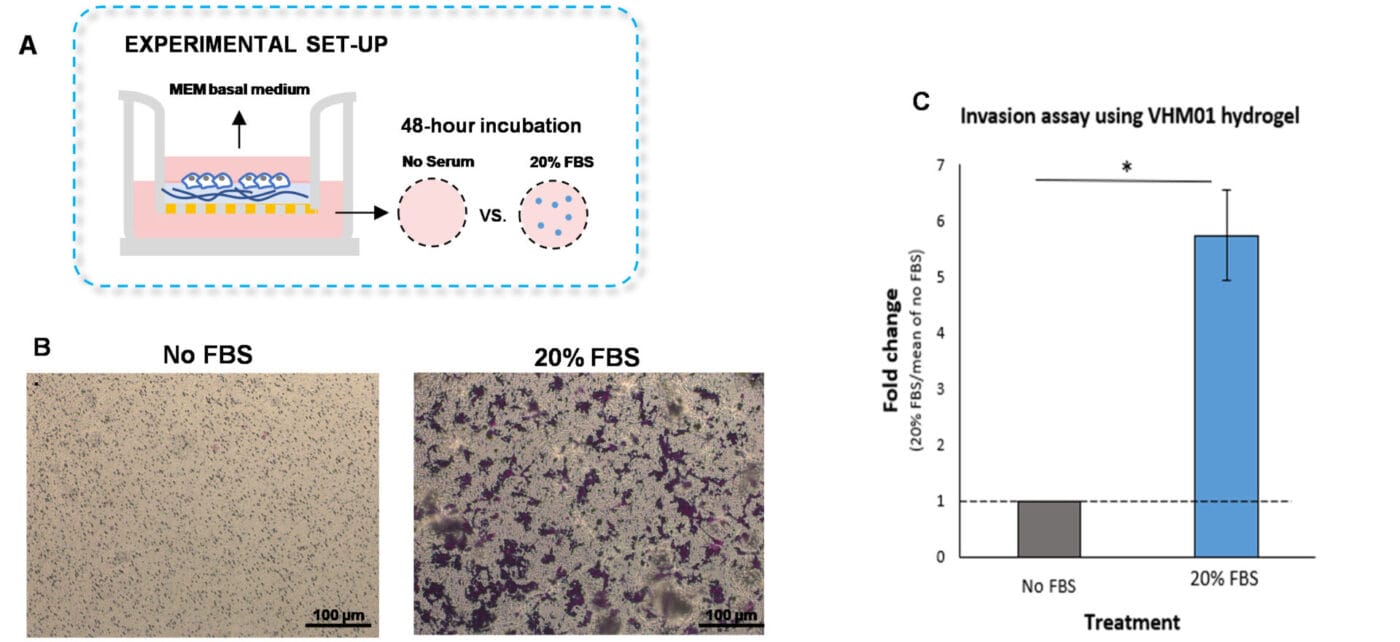
Figure 1. Invasion of U87-MG glioblastoma cells through VitroGel Hydrogel Matrix caused by a serum gradient.
A. Schematic representation demonstrating the invasion assay cell culture set-up. B. U-87 MG cell invasion was visualized by performing crystal violet staining followed by light microscopy. The images show the membrane inserts from the control group (No FBS) and 20% FBS conditions. Images were obtained with a Zeiss microscope at a 10X magnification. C. Fold change of U87-MG cell invasion between control and 20% FBS groups. The control group was normalized to 1. The asterisk (*) stands for p<0.05.
Evaluating cell invasion by embedding cytokines or chemokines into the VitroGel Hydrogel Matrix
- Ready-To-Use Hydrogel – Cell Invasion Assay Kit:
VitroGel® Cell Invasion Assay Kit (CAT # IA-VHM01-1P) - Insert: VitroGel® Hydrogel Matrix mixed with MEM basal medium with or without TGF-β1
- Outer well: MEM basal medium (without serum) or MEM with 20% FBS
- Cells: U87-MG cells (3 x 104 cells per insert)
- Cell incubation time: 24 hrs
The traditional invasion assay is frequently employed to examine cell invasion towards chemokines or cytokines located in the outer well. We adapted this method by embedding the cytokine, transforming growth factor (TGF)-β1, within the hydrogel instead of placing it in the outer well. Furthermore, the VitroGel Hydrogel Matrix was mixed with MEM basal medium supplemented with TGF-β1 (30 ng/mL) or with MEM basal medium in a 2:1 ratio (Figure 2A). The hydrogel mixture (100 µL) was added homogeneously to the inserts and allowed to solidify for 20 minutes at room temperature. Cells (3 x 104) were added on top of the hydrogel and the outer wells were filled with MEM basal medium or with MEM containing 20% FBS. The cultures were incubated for 48 hours at 37°C and then subjected to crystal violet staining to assess cell invasion.
To discern whether the cell invasion was due to TGF-β1 and not caused by the FBS in the outer wells, we established a control group in which the hydrogel was not supplemented with TGF- β1, but the outer well contained 20% FBS (Figure 2A). We observed enhanced outer wells, which is consistent with the findings shown in Figures 1B and 1C. However, the addition of TGF- β1 into the hydrogel increased cell invasion of U87-MG cells through the hydrogel compared to cells in which the outer wells contained serum-free medium or medium with FBS (Figures 2 B-C). These findings suggest that incorporating cytokines or chemokines into the VitroGel hydrogel is a great platform to evaluate chemotaxis better.
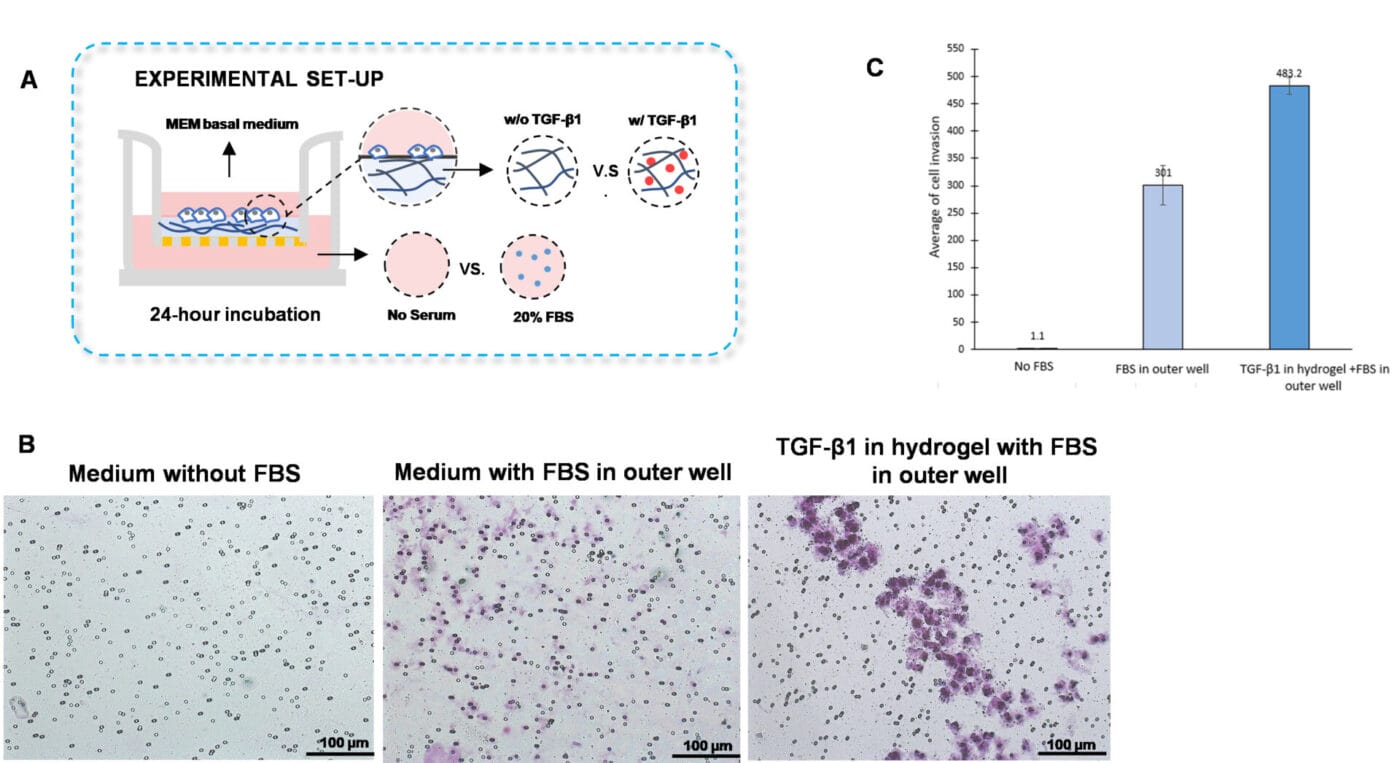
Figure 2. TGF-β1 inside of VitroGel hydrogel matrix induces invasion of U87-MG glioblastoma cells.
A. Visual representation of invasion assay setup. B. Light microscopy images demonstrating cell invasion in the different groups after crystal violet staining. Images were obtained with a Zeiss microscope at a 10X magnification. C. Mean of U87-MG cell invasion for each of the experimental conditions.
VitroGel® High-Concentration Cell Invasion Assay Kits (Tunable)
The effect of different hydrogel mechanical strengths in U87-MG glioblastoma cell invasion
- Tunable Hydrogel – High-Concentration Cell Invasion Assay Kit:
VitroGel RGD Cell Invasion Assay Kit (CAT # IA-HC003-1P) - Insert: VitroGel RGD hydrogel at 1:1, 1:3, and 1:5 dilution
ratios - Outer well: MEM basal medium (without serum) or MEM with 20% FBS
- Cells: U87-MG cells (3 x 104 cells per insert)
- Cell incubation time: 24 hrs
A unique property of VitroGel High-Concentration hydrogels is that the mechanical strength can be adjusted with VitroGel Dilution Solution for different in vitro and in vivo approaches. VitroGel High Concentration hydrogels can achieve mechanical strengths (elastic modulus) ranging from 10–4000 Pa, depending on the dilution ratio. Therefore, modulating the mechanical strength of the VitroGel High Concentration hydrogels is a method that can be adapted and applied to the traditional invasion assay.
To evaluate whether the hydrogel mechanical strength impacts cell invasion, we diluted VitroGel RGD with VitroGel Dilution solution using the following ratios: 1:1, 1:3, and 1:5 (Figure 3A). The hydrogel mixture was added to the inserts and incubated for 20 minutes at room temperature, and U87-MG glioblastoma cells resuspended in serum-free medium were placed on top of the inserts. The outer wells were covered with serum-free medium or medium with 20% FBS. Then, the cells were incubated for 24 hours at 37°C, followed by an examination of cell invasion using crystal violet staining.
The findings showed that increasing the dilution ratios of VitroGel RGD allows significant cell invasion through the hydrogel matrix when a chemoattractant, such as medium with serum, is placed on the outer wells (Figures 3 B-C). Specifically, we demonstrated that the 1:5 dilution of VitroGel RGD with VitroGel Dilution solution has the highest cell invasion through the hydrogel matrix, followed by the 1:3 and 1:1 dilution ratios (Figures 3 B-C). These findings suggest that adjusting the mechanical strength of the VitroGel RGD impacts cell motility towards an FBS gradient.
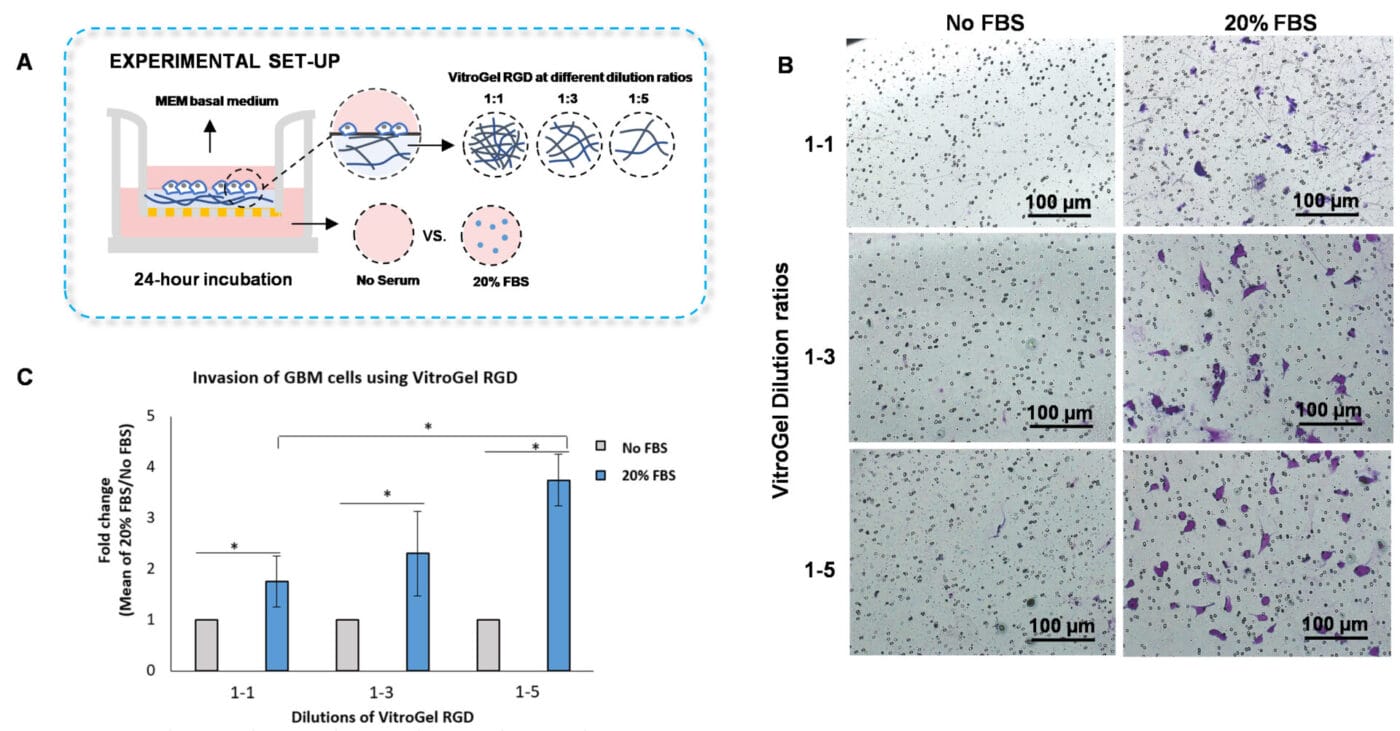
Figure 3. Invasion of U87-MG glioblastoma (GBM) cells using VitroGel RGD high-concentration hydrogel.
A. Schematic representation of experimental setup. B. Microscopy images showing U87-MG glioblastoma cell invasion through VitroGel RGD. The hydrogel was diluted with VitroGel Dilution solution in a 1:1, 1:3, and 1:5 ratio. Images were obtained with a Zeiss microscope at a 10X magnification. C. Fold change of cell invasion in the FBS group relative to the No FBS group for each dilution of VitroGel RGD. The No FBS group was normalized to 1. The asterisk denotes p < 0.05.
The effect of functional ligands within VitroGel High-Concentration hydrogels on U87-MG glioblastoma cell invasion
- Tunable Hydrogel – High-Concentration Cell Invasion Assay Kits:
VitroGel 3D Cell Invasion Assay Kit (CAT # IA-HC001-1P)
VitroGel RGD Cell Invasion Assay Kit (CAT # IA-HC003-1P)
VitroGel MMP Cell Invasion Assay Kit (CAT # IA-HC010-1P) - Insert: VitroGel 3D, RGD, or MMP hydrogel at 1:3 dilution ratios
- Outer well: MEM basal medium (without serum) or MEM with 20% FBS
- Cells: U87-MG cells (3 x 104 cells per insert)
- Cell incubation time: 48 hrs
VitroGel High-Concentration hydrogels are customized with various functional ligands for cell-based applications. Here, we demonstrate that VitroGel High-Concentration hydrogels are a suitable system for performing the traditional invasion assay. In this experiment, we aimed to determine whether our VitroGel High-Concentration hydrogels can influence U87-MG glioblastoma cell invasion towards a serum gradient. To examine how bio-functional ligands within VitroGel High-Concentration hydrogels modulate cell invasion, we used VitroGel 3D, an unmodified hydrogel; VitroGel RGD, a hydrogel modified with RGD peptides that support cell adhesion; and VitroGel MMP, which contains matrix metalloproteinases that enhance cell motility (Figure 4A).
To perform the cell invasion assay, the hydrogels were diluted with VitroGel Dilution Solution Type 2 in a 1:3 ratio. Then, the diluted hydrogel was mixed with MEM basal medium in a 4:1 ratio. The hydrogel mixture was added on the inserts and allowed to solidify for 20 minutes at room temperature. After this, the U87-MG glioblastoma cells were resuspended on MEM basal medium and incorporated on top of the hydrogels. The outer wells were replenished with MEM basal medium or MEM supplemented with 20% FBS. The cultures were incubated for 48 hours at 37°C. Following incubation, crystal violet staining was performed to evaluate cell invasion.
The findings showed that U87-MG cells cultured with VitroGel RGD had enhanced cell invasion compared to cells cultured in VitroGel MMP or VitroGel 3D (Figures 4B and 4C). Indeed, the modified hydrogels, VitroGel MMP, and VitroGel RGD, exhibited increased cell invasion relative to VitroGel 3D. Thus, the results suggest that the addition of bio-functional ligands to the hydrogels increases cell invasion towards a serum gradient.
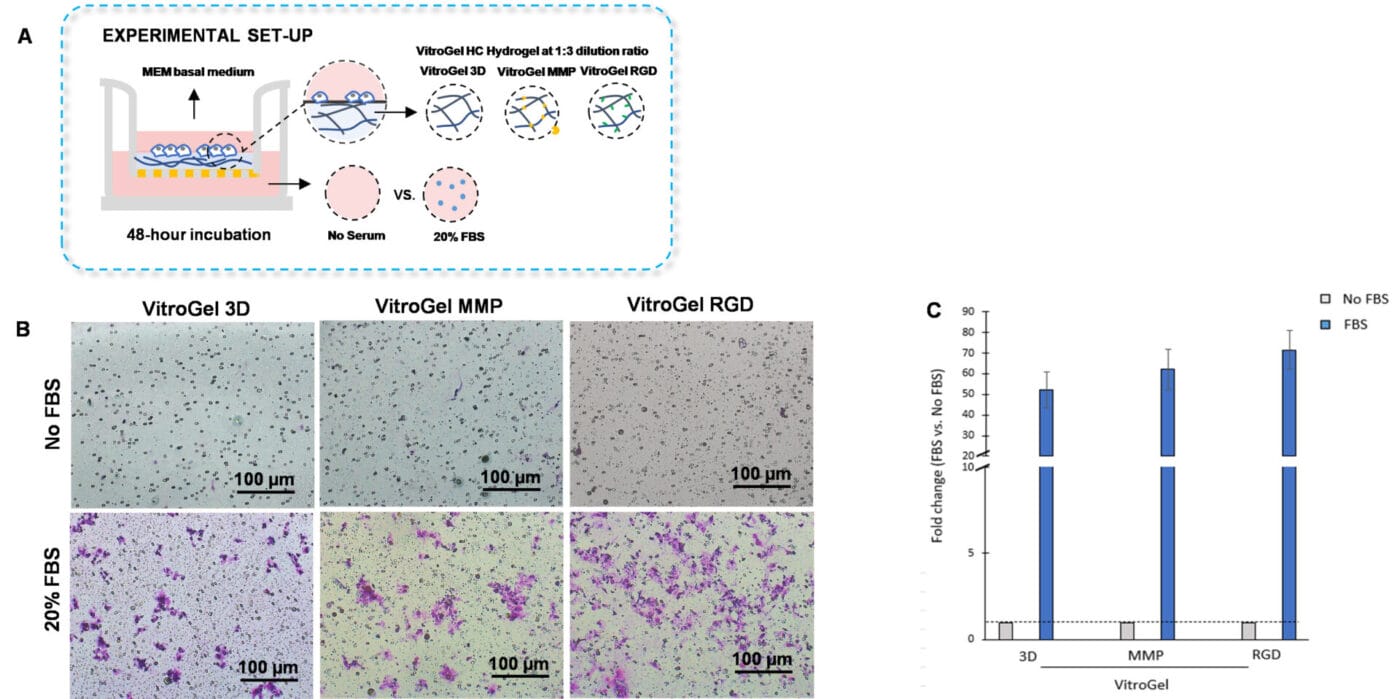
Figure 4. Bio-functional ligands inside hydrogel influence U87-MG glioblastoma cell invasion.
A. Schematic representation of experimental setup. Hydrogels were diluted with VitroGel dilution solution in a 1:3 ratio, placed in the insert, and allowed to solidify for 20 mins. B. Light microscopy images showing U87-MG glioblastoma cell invasion through VitroGel High-Cconcentration hydrogels with different bio-functional ligands. Images were obtained with a Zeiss microscope at a 10X magnification. C. Fold change of cell invasion in the FBS group relative to the No FBS group for each hydrogel.
The addition of cytokines, chemokines, and growth factors to VitroGel High-Concentration hydrogels in order to study cell invasion
- Tunable Hydrogel – High-Concentration Cell Invasion Assay Kits:
VitroGel 3D Cell Invasion Assay Kit (CAT # IA-HC001-1P )
VitroGel RGD Cell Invasion Assay Kit (CAT # IA-HC003-1P) - Insert: VitroGel 3D and VitroGel RGD hydrogels at 1:3 dilution ratio with TGF-β1 or without TGF-β1
- Outer well: MEM basal medium (without serum) or MEM with 20% FBS
- Cells: U87-MG cells (3 x 104 cells per insert)
- Cell incubation time: 48 hrs
VitroGel High-Concentration hydrogels are easy to use and can be tailored to various experimental applications, including the traditional invasion assay. Similar to the VitroGel Ready-to-use hydrogels, cytokines, chemokines, or growth factors can be incorporated into VitroGel High-Concentration hydrogels to assay cell invasion. Hence, we evaluated U87-MG glioblastoma cell invasion to a cytokine gradient by embedding TGF-β1 inside VitroGel 3D and VitroGel RGD High-Concentration hydrogels.
To examine U87-MG glioblastoma cell invasion towards a TGF-β1 gradient, we first performed a 1:3 dilution of each hydrogel with VitroGel dilution solution. Subsequently, the hydrogel mixture was combined with MEM basal medium or MEM basal medium supplemented with TGF-β1 (30 ng/mL) in a 4:1 ratio. The diluted hydrogel was added to the inserts and allowed to solidify for 20 minutes, followed by the addition of the cell suspension on top of the hydrogel. Serum-free medium or medium supplemented with 20% FBS was placed in the outer wells. The cells were incubated for 48 hours at 37°C. After that, cell invasion was assessed by performing crystal violet staining.
The addition of TGF- β1 inside the hydrogels stimulated U87-MG glioblastoma cell invasion. Moreover, we observed that embedding TGF- β1 in VitroGel RGD favored cell invasion compared to VitroGel 3D with TGF- β1. Consistent with the findings from Figures 3B and 3C, we showed that the medium with serum on the outer wells promoted cell invasion in the group in which the hydrogel did not contain TGF-β1. Altogether, the results demonstrate that cytokines, chemokines, or growth factors can be incorporated inside the VitroGel High-Concentration hydrogels to evaluate cell invasion.
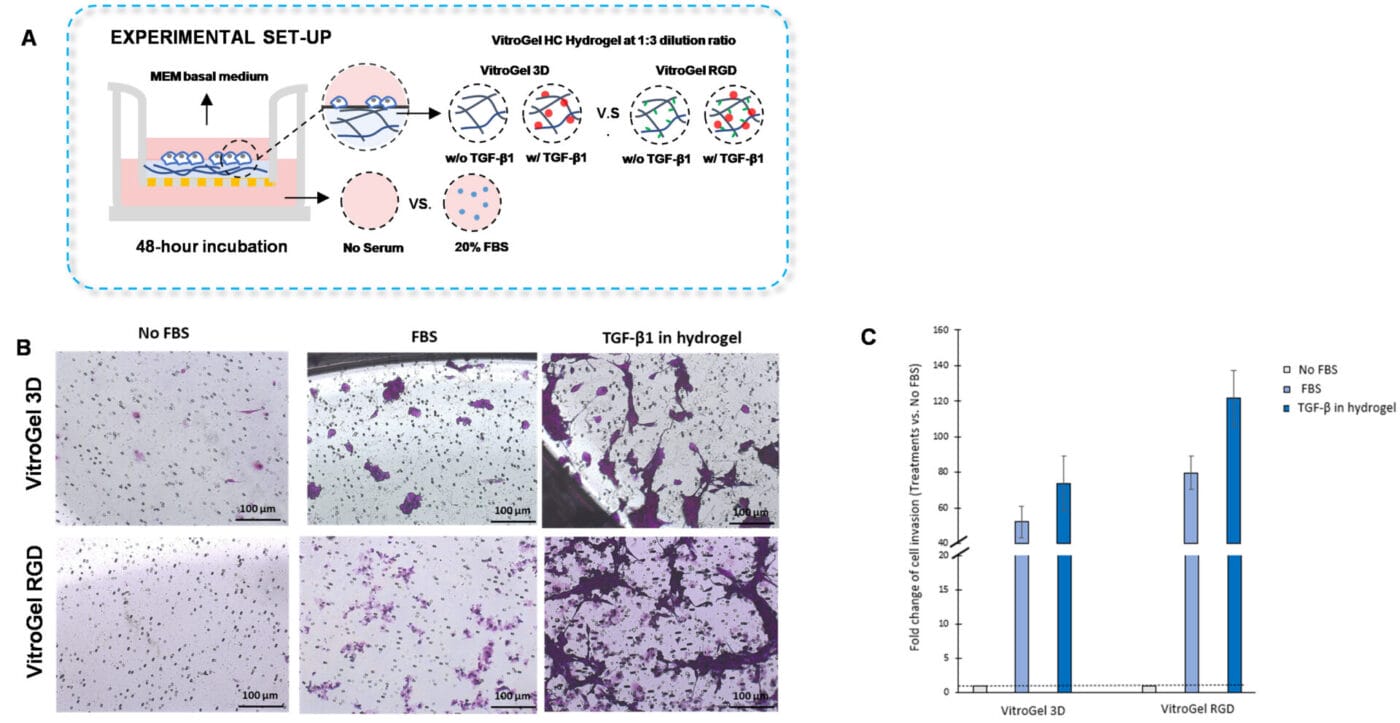
Figure 5. TGF-β1 inside VitroGel 3D and VitroGel RGD facilitates U87-MG glioblastoma cell invasion.
A. Visual representation of experimental setup. Cultures were incubated for 48 hours. B. Microscopy images demonstrating U87-MG glioblastoma cell invasion through VitroGel 3D and RGD. Each hydrogel was diluted with VitroGel Dilution solution in a 1:3 ratio and then combined with MEM basal medium or MEM with TGF-β1 (30 ng/mL) in a 4:1 ratio. Images were obtained with a Zeiss microscope at a 10X magnification. C. Fold change of cell invasion in the TGF-β1 in hydrogel and FBS groups relative to the No FBS group for each hydrogel. The No FBS group was normalized to 1.
Concluding Remarks
This study showcased that both the ready-to-use VitroGel® Cell Invasion Assay Kit and the tunable VitroGel “High-Concentration” Cell Invasion Assay Kits are great tools for invasion assay applications. Unlike animal-based ECMs, the components of VitroGel hydrogels are well-characterized, a fact that enhances reproducibility between experiments and minimizes undesirable effects caused by unknown factors. In addition, VitroGel hydrogels are not temperature sensitive and, as a result, they are easy to use compared to other commercially available products. Importantly, the synthetic nature of VitroGel hydrogels allows researchers to modify the matrix by incorporating chemokines, cytokines, growth factors, and pharmacological agents to evaluate their effect on cell invasion and cancer metastasis.
The data illustrated in this application note established that both the ready-to-use VitroGel® Hydrogel Matrix and the tunable VitroGel® “High-Concentration” hydrogels can be used for the traditional invasion assay. The VitroGel Hydrogel Matrix is an excellent choice for researchers whose experiments do not require adjusting the hydrogel’s mechanical strength or a specific bio-functional ligand to study cell invasion. Conversely, the VitroGel High-Concentration hydrogels are tunable; therefore, when combined with VitroGel Dilution Solution, the mechanical strength of the elastic modulus can be adjusted up to 4000 Pa. Our findings showed that increasing the dilution ratio of the High-Concentration VitroGel hydrogel (VitroGel RGD with VitroGel Dilution Solution) enhanced glioblastoma cell invasion, indicating that the mechanical strength impacts cell invasion. Furthermore, VitroGel High Concentration hydrogels are customized with specific bio-functional ligands such as MMPs, RGD, and others, allowing the evaluation of these factors in cell invasion. This study demonstrated that bio-functional ligands in the hydrogel influence glioblastoma cell invasion.
As previously mentioned, a novel and unique application that can be employed with the ready-to-use VitroGel® Cell Invasion Assay Kit and the tunable VitroGel® High Concentration Cell Invasion Assay Kits is the addition of cytokines, chemokines, growth factors, or pharmacological agents. Therefore, we used this approach to evaluate glioblastoma cell invasion towards a TGF-β1 gradient inside the hydrogels. Our findings depicted that TGF-β1 in the hydrogels stimulates glioblastoma cell invasion, thus demonstrating that incorporating factors inside VitroGel hydrogels is a powerful method to evaluate chemotaxis. Altogether, the VitroGel-Based Cell Invasion Assay Kits comprise a potent and flexible system that can be suited to myriad invasion assay applications.
References
1. Mahauad-Fernandez WD, Okeoma CM (2018). B49, a BST-2-based peptide, inhibits adhesion and growth of breast cancer cells. Sci Rep 8, 4305. https://doi.org/10.1038/s41598-018-22364-z
2. Di Donato, M, Cernera G, Migliaccio A, Castoria G (2019). Nerve Growth Factor induces proliferation and aggressiveness in prostate cancer cells. Cancers, 11, 784. https://doi.org/10.3390/cancers11060784
3. Ramos RI, Bustos MA, Wu J, Jones P, Chang SC, Kiyohara E, Tran K, Zhang X, Stern SL, Izraely S, Sagi-Assif O, Witz IP, Davies MA, Mills GB, Kelly DF, Irie RF, Hoon DSB (2020). Upregulation of cell surface GD3 ganglioside phenotype is associated with human melanoma brain metastasis. Mol Oncol 14, 1760–1778. https://doi.org/10.1002/1878-0261.12702