White Papers
Comparing the Xeno-Free VitroGel to Animal-Based Matrigel for Xenograft Studies
White Paper
Hera Bioscience, Lexington, KY 40505
TheWell Bioscience, North Brunswick, NJ 08902


Introduction
A cell-derived xenograft (CDX) model is the gold standard for anti-cancer therapies. The response of xenografts obtained in immune-deficient animals in drug testing is comparable to that in clinical practice, which makes xenograft models robust research tools in oncology1. In addition, over the past decades, patient-derived xenograft (PDX) models have emerged for precision medicine to match drug-to-patient or groups of patients to better response efficacy2.
Hydrogels used as an injectable carrier play a critical role in xenograft applications. Matrigel® is one type of hydrogel, which is an animal-based extracellular matrix (ECM) that has been widely used for traditional xenograft applications. However, the use of this type of biologically derived matrices has batch-to-batch consistency issues and can also periodically lead to the transmission of disease or negative immunological reactions. The temperature sensitivity of this material requires special shipping conditions as well as time-consuming and unique protocol setup. For example, if the material reaches room temperature, it begins to solidify and causes the needle to clog during the injection. Additionally, the supply chain of these types of biologically derived materials is more likely to experience inventory disruption in the market due to the vulnerability of the supply source and is subject to significant delays, as seen during the COVID-19 pandemic.
In this application, we use TheWell Bioscience’s VitroGel® Hydrogel Matrix (SKU: VHM01), one of the most popular ready-to-use VitroGel hydrogel, to make a side-by-side comparison with Matrigel for the xenograft application. Two human-derived cancer cell lines (H2170 lung cancer cells and PC3 prostate cancer cells) were mixed with VitroGel and Matrigel, respectively, and xenografted into Hera BioLabs’ SRG Rat model, as well as additional mouse models. The results show that VitroGel Hydrogel Matrix can support a higher tumor formation rate and fast cell growth kinetics with a lower or comparable standard deviation compared to Matrigel. See Figure 1 for protocol overview.
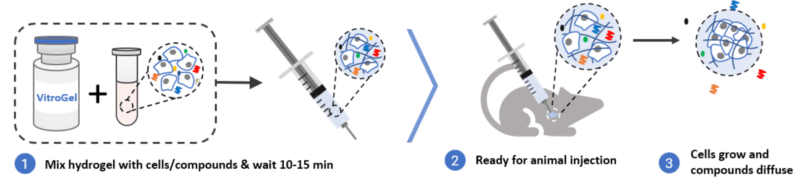
Product Overview
About VitroGel
TheWell Bioscience’s VitroGel is a superior alternative to Matrigel as an injectable carrier for xenograft, targeted delivery, controlled release, and cell therapy for tissue engineering.  As an efficient hydrogel system, VitroGel carries cells/tissue into the target injection site, maintains excellent cell retention at the injection location, and supports cell growth after delivery. Unlike animal-based ECM, which requires delicate cold temperature operation, VitroGel is an easy-to-use hydrogel solution with the operation setup and cells/compounds hydrogel mixture at room temperature. After a simple, quick, and easy preparation setup, it is ready for injection and maintains long injectable status.
As an efficient hydrogel system, VitroGel carries cells/tissue into the target injection site, maintains excellent cell retention at the injection location, and supports cell growth after delivery. Unlike animal-based ECM, which requires delicate cold temperature operation, VitroGel is an easy-to-use hydrogel solution with the operation setup and cells/compounds hydrogel mixture at room temperature. After a simple, quick, and easy preparation setup, it is ready for injection and maintains long injectable status.
About SRG Rat
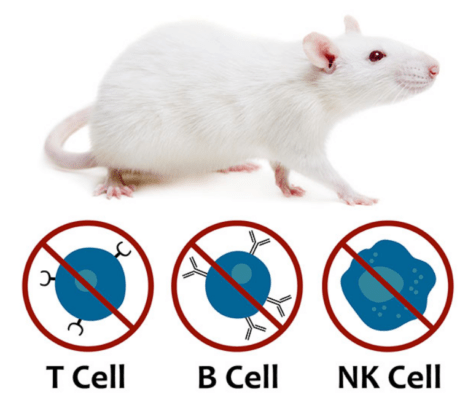
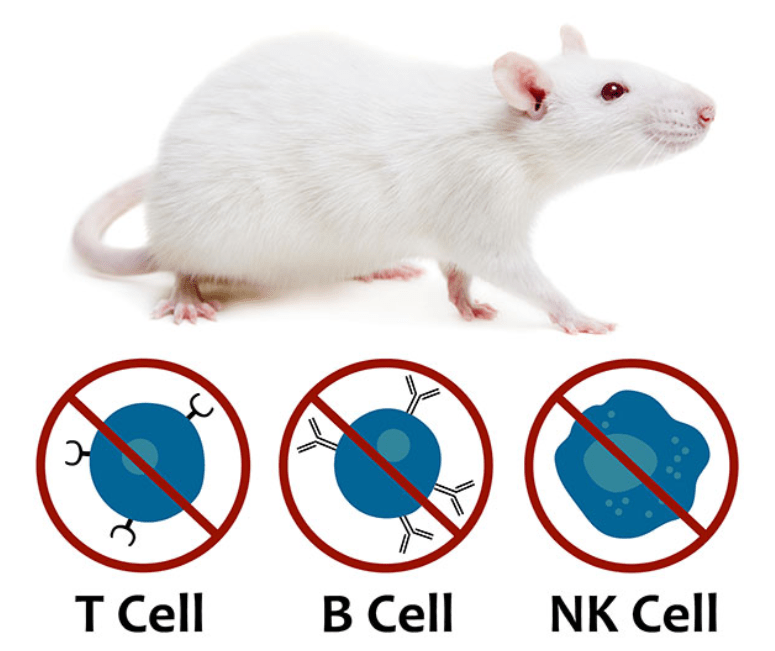
Hera BioLabs offers preclinical oncology expertise with the SRG rat model. The SRG rat is a SCID model created through knockout mutations in the Rag2 and Il2rgamma genes that result in a loss of mature B, T, and NK cells. The SRG Rat has an immunodeficient phenotype ideal for the engraftment of human tissue. This severe immunodeficiency, combined with a larger organism size for tumor growth, makes the SRG rat an ideal alternative research model to mice in certain preclinical applications.
Materials and Methods
Materials
Either H2170 lung cancer cells or PC3 prostate cancer cells were used in this xenograft study. SRG (Sprague Dawley-Rag2em2heraIl2rgem1hera/HblCrl immunodeficient) rats from Hera BioLabs were used as the hosts. The xeno-free VitroGel Hydrogel Matrix and animal-based Matrigel were used as injectable carriers to make the side-by-side comparison.
Preparing Hydrogel-Cell Mixture for Animal Injection
H2170 cells and PC3 cells were harvested and resuspended in PBS without additional supplement. Matrigel was thawed and kept in an ice bucket; VitroGel Hydrogel Matrix was brought to room temperature before mixing. The hydrogel solutions and cell suspension were mixed at 1:1 (v/v) ratio, and a 500 µL hydrogel-cell mixture was subcutaneously injected into the SRG rat host. The numbers of cells injected were 1 x 107 cells for H2170 cells and 5 x 106 for PC3 cells. Please check Table 1 for more details.
Table 1. Xenograft Sample preparation using VitroGel system
| Cell Type | Medium | Mixing ratio with VitroGel (V/V) | # Of cells injected | Host | Injection volume | Injection site | Incubation time |
| H2170
(Human lung cancer) |
PBS | 1:1 | 1 x 107 | SRG rat | 500 µL | Subcutaneous injection | 7 weeks |
| PC3
(Human prostate cancer) |
PBS | 1:1 | 5 x 106 | SRG rat | 500 µL | Subcutaneous injection | 6 weeks |
For detailed VitroGel sample preparation protocols, please check: VitroGel Injection Protocol – Xenograft In Vivo Applications
Injection and Tumor Progression
Injections into rats were performed in replicate with 3–5 rats. Tumor volumes were measured every two- or three days post-injection.
Results
Tumor volumes induced by injection with either VitroGel Hydrogel Matrix or Matrigel over approximately seven weeks are shown in Figure 2. For human lung cancer cells (H2170), the tumor formation rate (i.e., the cell growth rate) in VitroGel was 70% higher than that in Matrigel after 50 days post-injection. The mean growth rates for human prostate cancer cells (PC3) were nearly identical in the two matrices. The standard deviation across the host samples for the H2170 cell type was much lower in VitroGel than in Matrigel, and for the PC3 cell type, the standard deviation in VitroGel was a similar standard deviation in Matrigel.
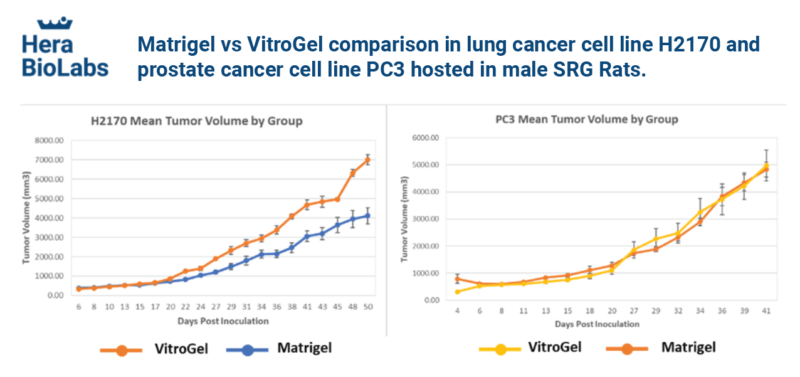
Discussion
The data demonstrate that VitroGel can support the growth of xenografted human lung and prostate cancer tissues at least as well as Matrigel in a rodent host. The former system is biocompatible without showing a toxic or inflammatory response, as demonstrated by our internal animal safety studies5. In fact, the growth rate of H2170 cells was significantly higher in VitroGel, portending excellent results in a variety of xenograft systems. Moreover, the variation in growth rates as a function of time was noticeably lower for VitroGel for both cell types tested. This suggests that the synthetic hydrogel system can deliver more consistent results and long-term injectable properties. The VitroGel system makes it possible to do a whole group of animal injections with one hydrogel-cell mixture preparation, which reduces the operational error observed with Matrigel’s repeated sample preparation with an animal-based hydrogel system. Potentially, scientists can use fewer animals (increasing cost-efficiency), devote less time by switching to VitroGel, and still obtain comparable, if not better, results to those obtained with traditional ECM sources.
More about VitroGel
TheWell Bioscience’s VitroGel® is a superior alternative to animal-based and plant-based ECM as an injectable carrier for xenograft, targeted delivery, controlled release, and cell therapy for tissue engineering. As an efficient hydrogel system, VitroGel carries cells/tissue into the target injection site and maintains excellent cell retention at the injection location and supports cell growth after delivery. Unlike animal-based ECM, which requires delicate cold temperature operation, VitroGel is an easy-to-use hydrogel solution with the operation setup and cells/compounds hydrogel mixture at room temperature, see figure 1. After a simple, quick, and easy preparation setup, it is ready for injection and maintains injectable status for hours at room temperature or at 37°C.
Because VitroGel has a unique rheological property that can maintain an excellent injectable status (reversible sol-gel transition status) for hours after mixing with cells, scientists can prepare samples in bulk and continue to inject without worrying about needle clogging. The xeno-free bio-functional hydrogel system has many other advantages (figure 3). During animal safety studies, VitroGel demonstrated biocompatibility without showing a toxic or inflammatory response. Researchers have full control over the ECM supplements to optimize and boost cell growth. In addition, VitroGel is not constrained by supply chain issues and is manufactured with batch-to-batch consistency for reproducible lots.
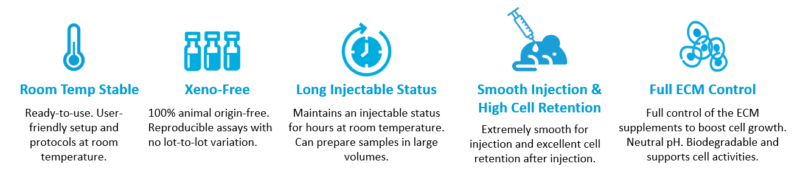
TheWell Bioscience offers a repertoire of hydrogels, both ready-to-use, and tunable high-concentration hydrogels. The tunable high concentration hydrogels give the ability to adjust the mechanical strength of the ECM, which opens the door for many new assays to study: the cell-to-material interactions regarding gel strengths, multi-functional ligands for many cell types, degradability, and the growth factors within the hydrogel matrix that animal-based material simply cannot do.
References
- Mattern, , Bak, M., Hahn, E. W., & Volm, M. (1988). Human tumor xenografts as model for drug testing. Cancer Metastasis Reviews, 7(3), 263–284. https://doi.org/10.1007/BF00047755
- Risbridger, P., Lawrence, M. G., & Taylor, R. A. (2020). PDX: Moving Beyond Drug Screening to Versatile Models for Research Discovery. Journal of the Endocrine Society, 4(11). https://doi.org/10.1210/jendso/bvaa132
- Perez M., Kearney J.F., & Yeh J.J. (2021). The PDAC extracellular matrix: A review of the ECM protein composition, tumor cell interaction, and therapeutic strategies. Frontiers in Oncology, 11: 751311. https://doi.org/10.3389/fonc.2021.751311.
- Badylak S.F. (2004). Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transplant Immunology, 12 (3–4): 367–377. https://doi.org/10.1016/j.trim.2003.12.016.
- https://www.thewellbio.com/applications/in-vivo/xenograft-pdx-cdx/
Links To Products Used
All product names, logos, brands, trademarks and registered trademarks are property of their respective owners.