Application Notes
3D Cell Culture of Human Colon Cancer Cells (HCT116) on VitroGel® System
Application Note
TheWell Bioscience Inc.
Introduction
In vitro assays have become increasingly popular in drug discovery applications because of their high throughput functionality and cost-effectiveness in conducting optimization and preclinical validation. Three-dimensional (3D) culture assays have become especially popular because compared to traditional two-dimensional (2D) cell culture, 3D culture provides a more physiologically relevant environment (1-4). Comparative studies have shown that 2D cultures lose tissue-specific architecture, causing changes in mechanical and biochemical cues, and interrupting cell-cell or cell-matrix interactions (5). These differences become exceedingly important in drug development. While many drugs exhibit success in 2D cultures, this may not be translatable in vivo because the 3D environment of the great majority of cells in the body may make it difficult for the drugs to attack all cells (7).
TheWell Biosciences VitroGel® System is a tunable hydrogel system that is ready to use. It can be used for both 2D and 3D systems, and in both in vitro and in vivo applications. The hydrogel is adjustable based on the concentration of the VitroGel and the density of cells and can be adjusted to fit any cell type and mixed with any culture medium to generate the scaffold. The VitroGel system has been used to create 3D structures of many different cell types for various applications. For example, a recent study analyzing the efficacy of a novel anti-cancer peptide found that breast cancer cells form spheroid groups with other cells in culture (6). Addition of their novel peptide resulted in disruption of the spheroid, suggesting it could inhibit adhesion-independent cell growth, a conclusion that may not have been elucidated in a two-dimensional environment.
In this study, we evaluate the viability and survival of HCT116 cells in the VitroGel system for 2D coating, 3D culture, and drug testing (8). Both VitroGel 3D and VitroGel 3D-RGD (the RGD peptide-modified hydrogel system for better cell adhesion) were used in this study. HCT116 is a line of human colorectal carcinoma cells commonly used in colon cancer studies and therapeutic drug development. These studies establish the VitroGel system as a viable means by which to study drug development using HCT116 cells as a model for colorectal cancer.
Materials and Methods
Cell Culture: HCT116 cells were maintained in T-25 flask with McCoy’s 5a with 10% FBS and 1x pen-strep. Cells were passaged when the cultures reached ~80% confluence. For 3D cell culture and drug testing, VitroGel 3D-RGD (TWG002, TheWell Bioscience) was used, and for 2D cultures, both VitroGel 3D (TWG001, TheWell Bioscience) and VitroGel 3D-RGD were used, according to the user handbook (8), as described below.
2D Coating Culture Method
Hydrogel solution was mixed with 1X PBS at 1:1 ratio, then transferred to a 24-well culture plate at 250 µL per well. To stabilize the hydrogel, it was allowed to sit at room temperature for 20 min. Cell suspension (about 150K to 200K cells/mL, 250 µL/well) was added on the top of the hydrogel. On day 2, an additional 200 µL complete cell culture medium was added to the top medium. After that, the top medium was changed according to TheWell Bioscience user handbook (8), 60-70% of top medium changed every other day.
3D Culture Method
Hydrogel solution was diluted with 0.5X PBS solution at 1:0, 1:1 and 1:2 ratio (v/v). The cell suspension was mixed in at a 2:1 ratio (2mL diluted hydrogel solution with 1 mL cell suspension, final cell concentration: 150K to 200K cells/mL ). Hydrogel/cell mixture was added to a 24-well plate at 250 µL per well. The hydrogel was then allowed to stabilize at room temperature for 20 minutes. After stabilization, 250 uL complete cell culture medium was added on the top of the hydrogel. On day 2, an additional 200 µL complete cell culture medium was added to the top medium. After that, the medium was changed according to TheWell Bioscience user handbook (8), 60-70% of top medium changed every other day.
Drug Testing
HCT116 cells were seeded at 500K cells/mL on top of chambered coverglass slides in VitroGel 3D-RGD (1:1 0.5X PBS dilution, 2:1 mixing) for 3 days respectively. On Day 4, the anti-cancer drug (5-fluorouracil, Sigma) was added on the top of both 2D and 3D cultured cells at the concentrations of 0 µM, 10 µM, 25 µM, 50 µM. Cells were treated with the drug for 3 days. On Day 7, the top medium was removed, and the live/dead assay was performed according to manufacturer instruction (Thermo Fisher R37601, Live/Dead® Cell Imaging Kit). Quantification of Live/Dead Staining was processed using Image J.
Immunofluorescent (IF) Imaging
For IF imaging, cells were grown in chambered coverglass slides in lieu of 24-well plates. Each chamber holds 125 uL hydrogel/cell mixture. Cells were fixed and stained by using the Image-iT® Fixation/Permeabilization Kit, ActinGreen™ 488 ReadyProbes® Reagent and NucBlue® Fixed Cell ReadyProbes™ Reagent (ThermoFisher) according to manufacturer protocols. The images were taken using a Leica TCS SP8 scanning confocal microscope with z-stack function and processed by LAS X software and ImageJ.
Statistical Analysis
Statistics were done using an unpaired t-test comparing the drug concentrations to 0µM as a control. P-values less than 0.05 are considered to be significant (*), p-values less than 0.005 are very statistically significant (**) and values less than 0.001 are extremely statistically significant (***).
Results
HCT116 Cells Can Grow On Both 2D Coating and 3D VitroGel Systems
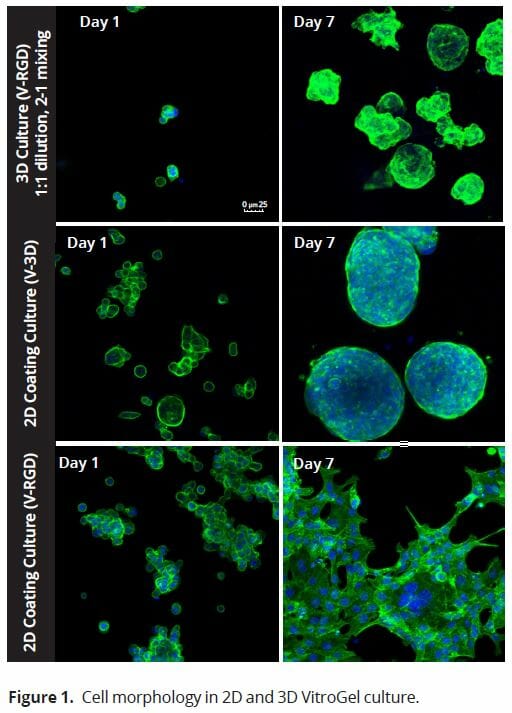
We evaluated HCT116 cells using both versions of the VitroGel system (Figure 1): the standard VitroGel-3D system, which is ideal for suspension cells, and the RGD-modified version, which is contains the RGD peptide modification, a peptide present in all major extracellular matrix proteins, and which can promote cell adhesion to biomaterials such as hydrogels (9). Cells were evaluated 24 hours after seeding cells and again 7 days later. When using the 2D coating method, in which the VitroGel hydrogel is allowed to set before cells are seeded, cells adopted a different morphology depending on which version of the VitroGel system was used. In the VitroGel 3D, cells acquired a spheroid shape after 24 hours and continued to increase in size out to 7 days. Like the standard VitroGel 3D, cells on VitroGel 3D-RGD also began to form spheroid cell clusters after 24 hours. However, unlike the standard hydrogel, cells spread out after 7 days, adopting a morphology reminiscent of the 2D culture on standard treated culture plastic. When using the 3D cell culture method in the VitroGel 3D-RGD system, in which cells are seeded into the hydrogel prior to the setting of the gel, HCT116 cells are found to distribute homogenously within the hydrogel. This process seems to begin more slowly; after 24 hours, cell clusters are very small, only 2-3 cells per cluster. By day 7, the clusters grow into smaller, more uniformly sized spheroids.
The strength of the hydrogel can affect its mechanical properties, and thus cell viability and response within the hydrogel. We analyzed growth potential (Figure 2) and viability (Figure 3) of HCT116 cells in three different hydrogel strengths (concentrations): 1:0 dilution (mostly hydrogel), 1:1 dilution (hydrogel/PBS) and 1:2 dilution. After 7 days, the 1:1 hydrogel dilution allowed for big colonies, while other dilutions only allowed for small cell clusters/colonies with abnormal morphology. Analysis of cell viability by live/dead assay showed that there was no significant cell death in any of the hydrogel dilutions, despite reduced colony size. Although the cells in the 1:0 and 1:2 scaffold dilutions aren’t exhibiting many dead markers, their morphology indicates they may be dying if not already dead. In the 1:1 dilution, there are more cells, and although they have a flatter morphology commonly associated with poor cell health, it is thought that the increased cell-matrix interaction allowed in scaffolds prevents the spheroid morphology seen in ultra low-adhesion plates used in 2D culture. This morphology might indicate a good cell-matrix interaction which could be good to mimic in vivo situation, and also suggests that the optimal scaffold dilution is 1:1 scaffold:PBS.
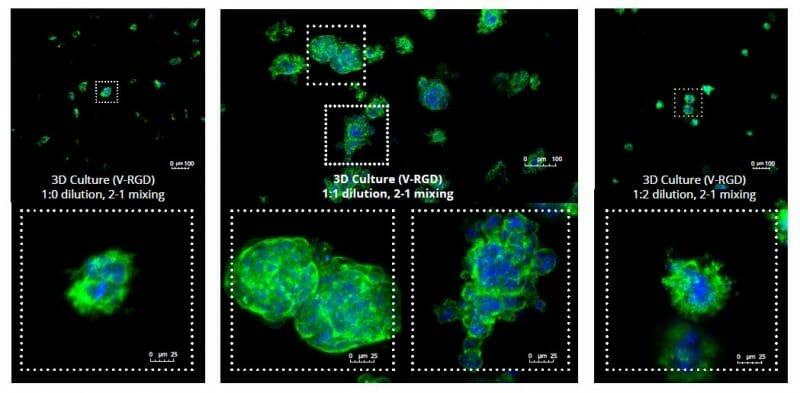
Figure 2: 3D cell culture of HCT116 Cell in different hydrogel concentrations (enlarged cells showing at the bottom).
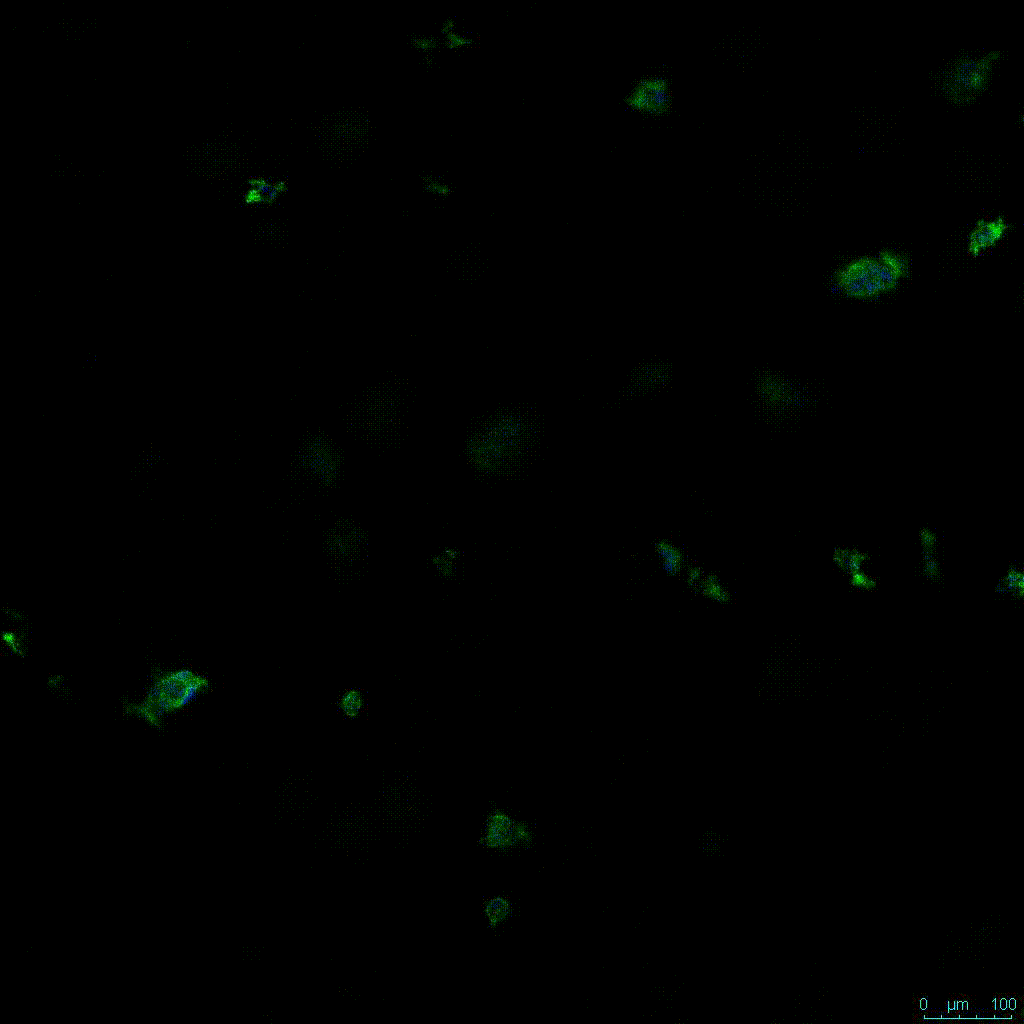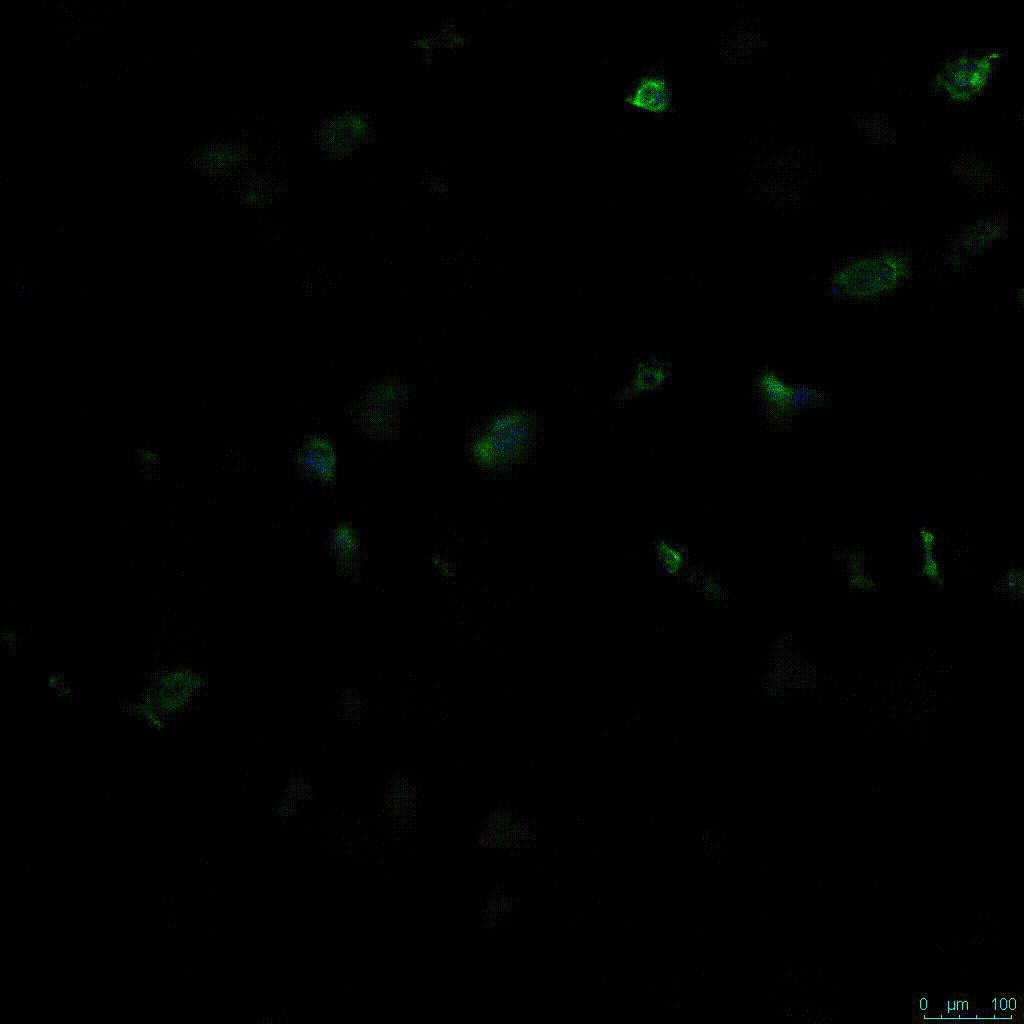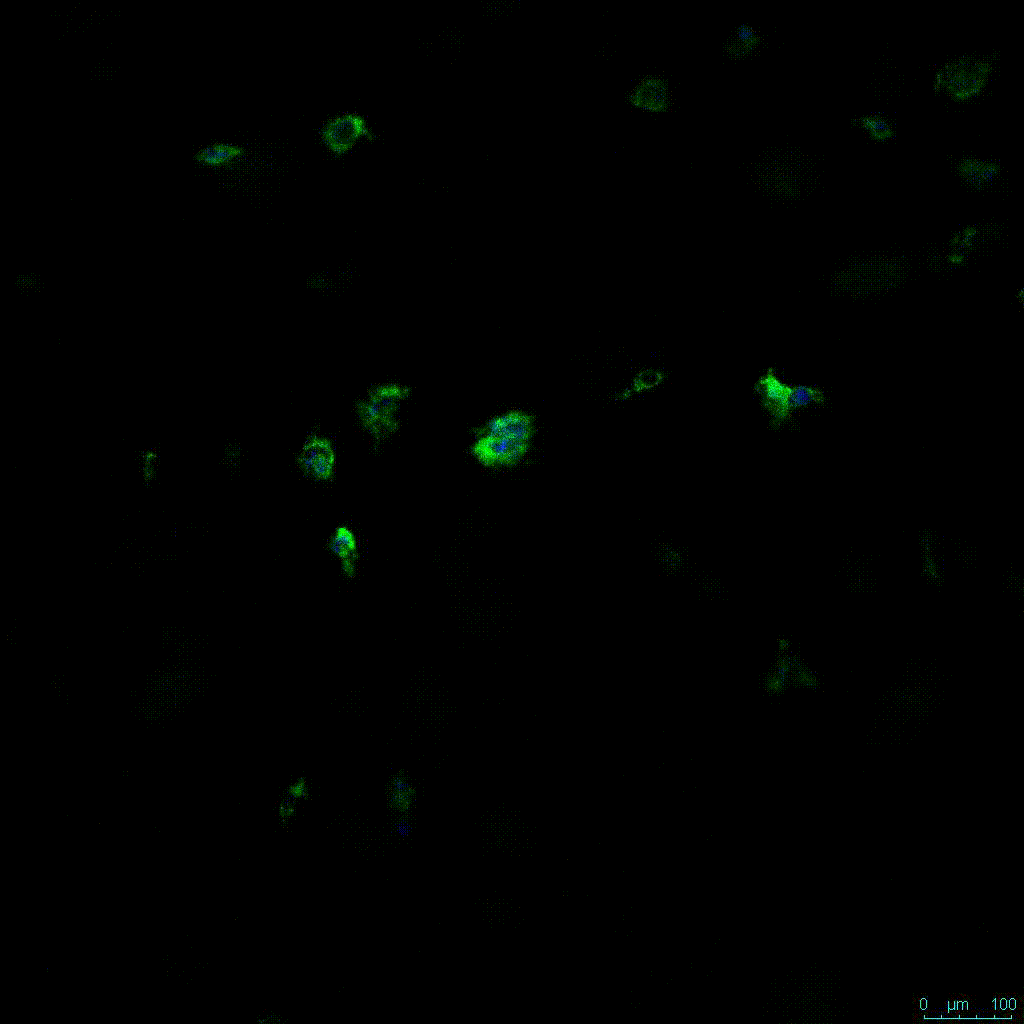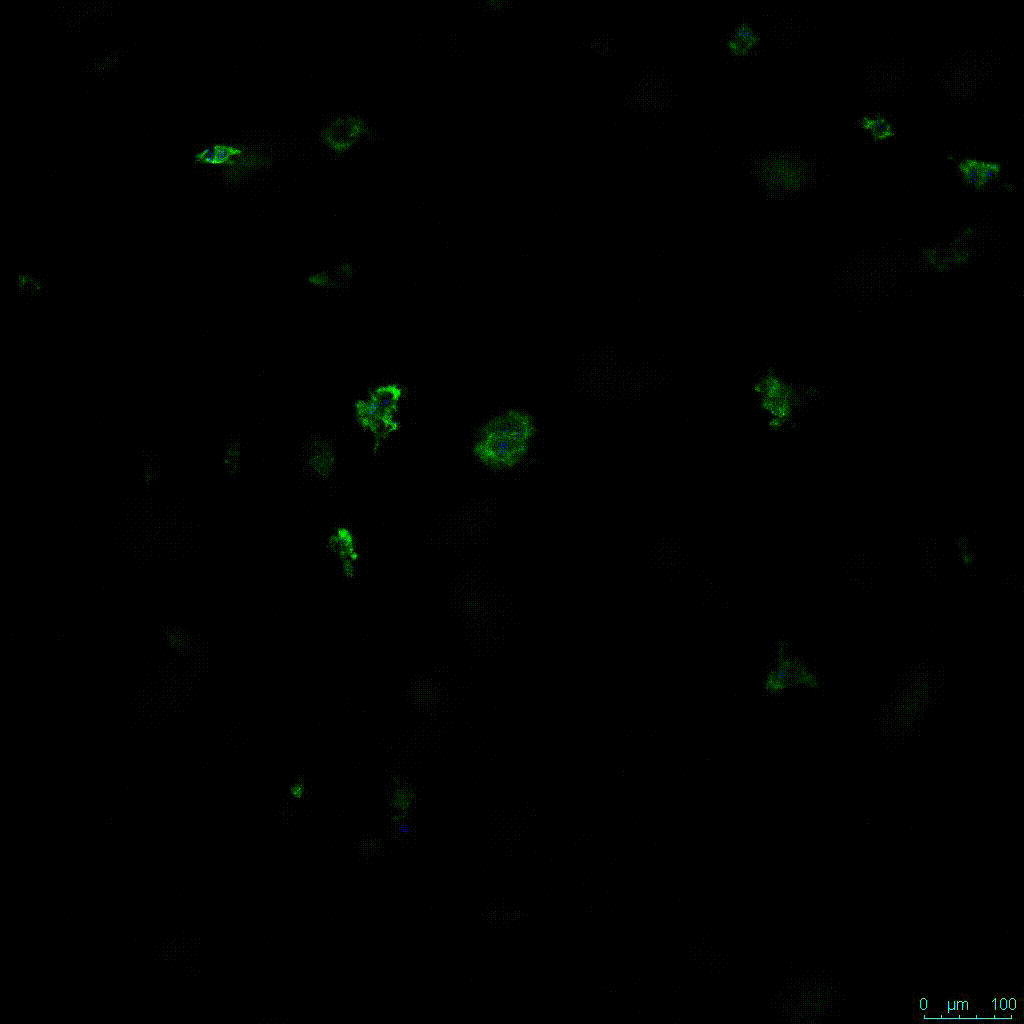
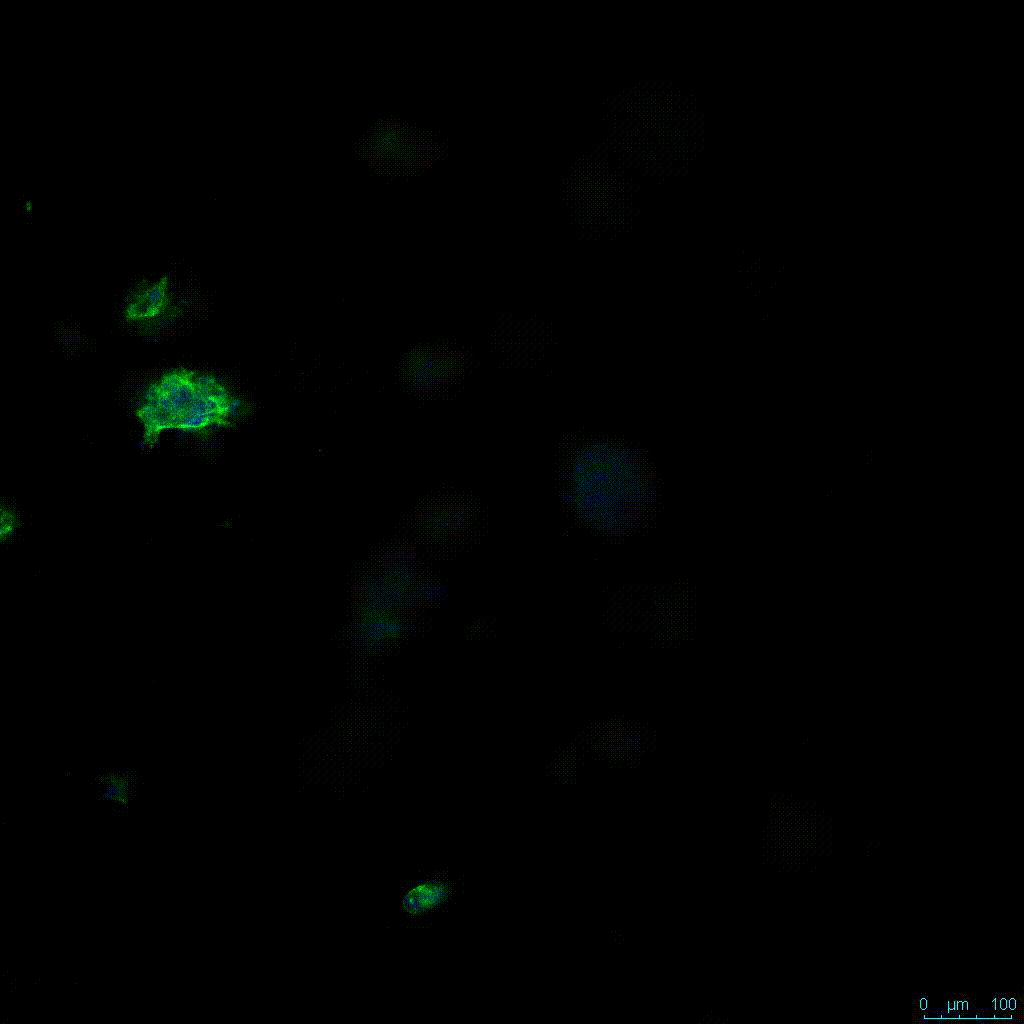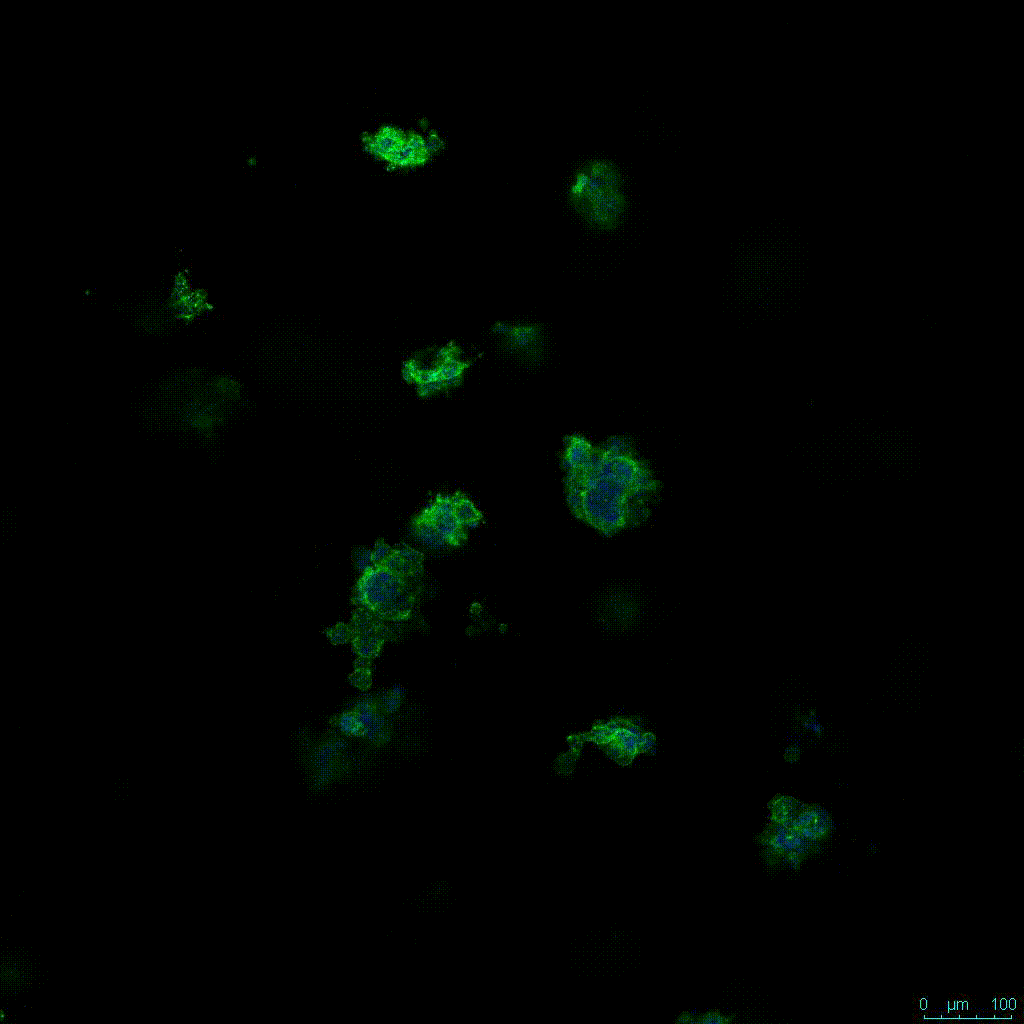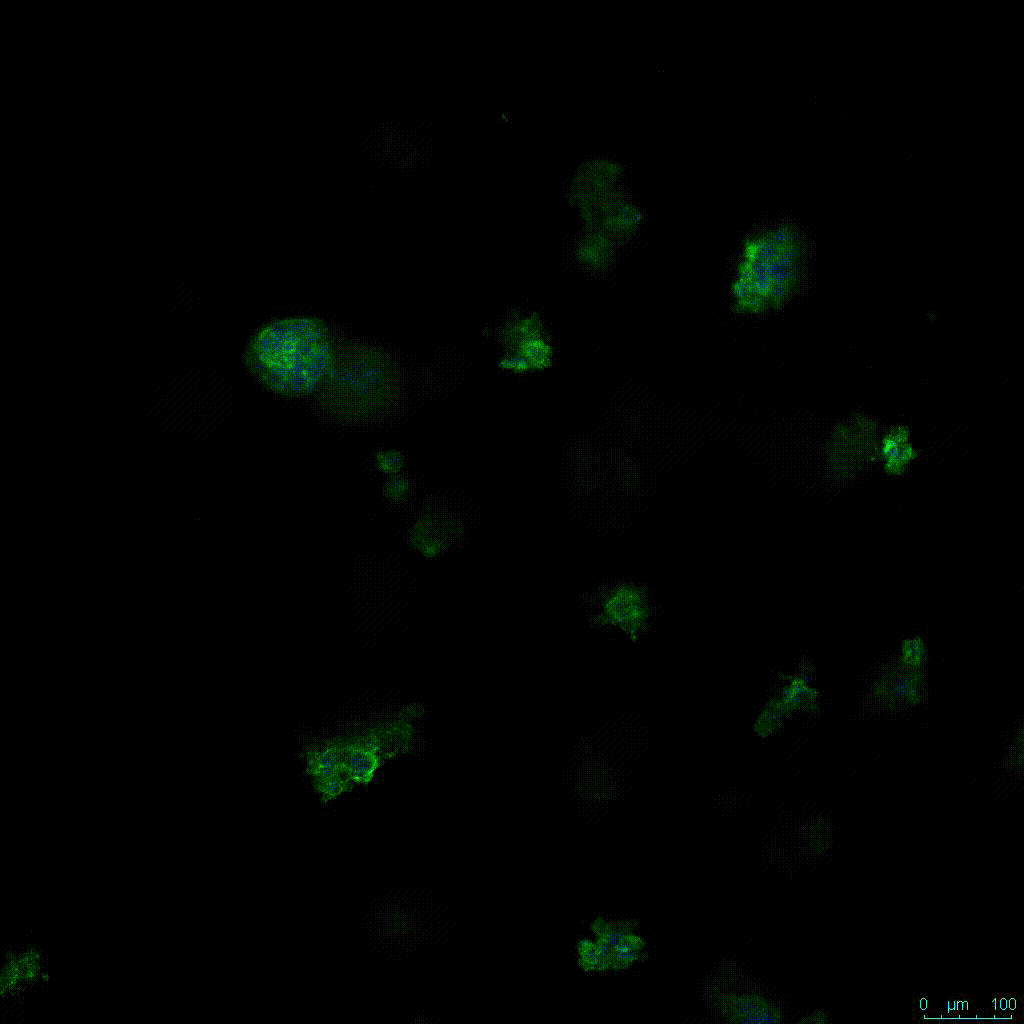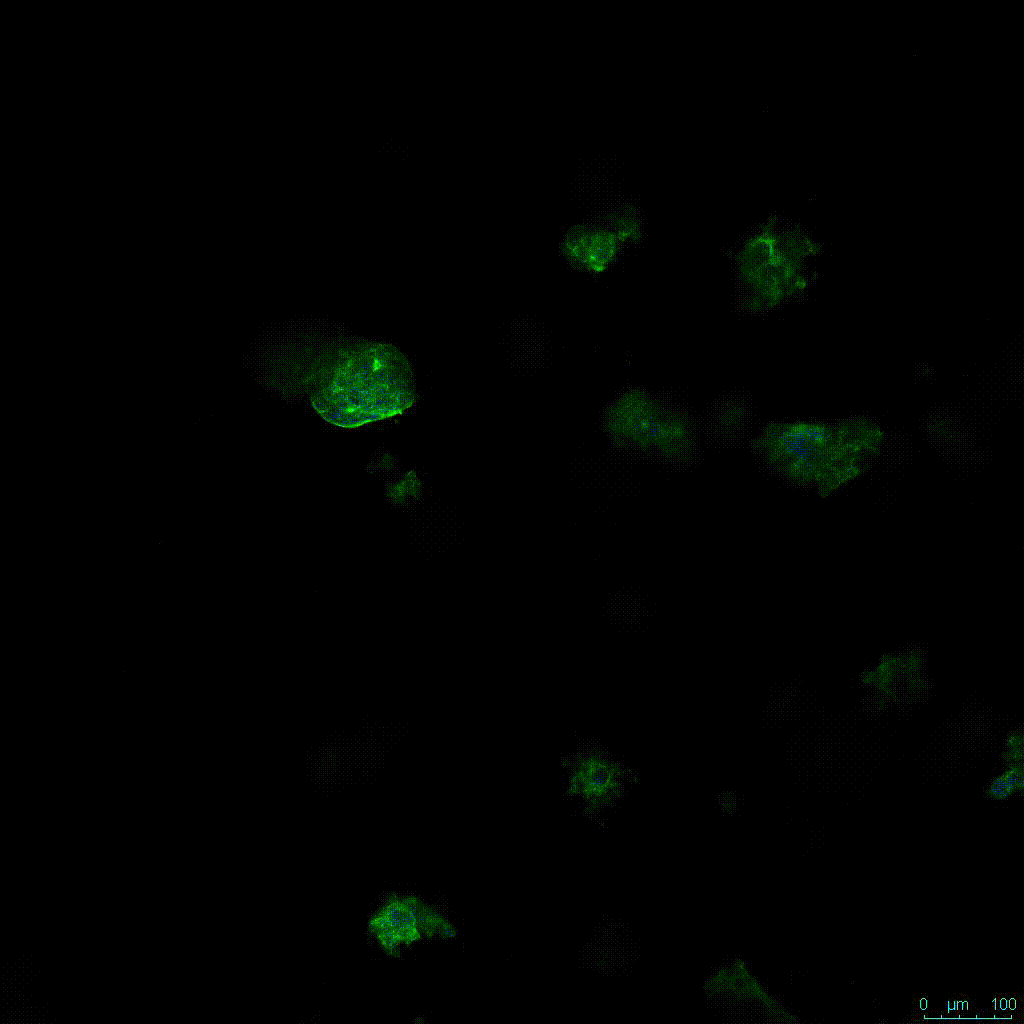
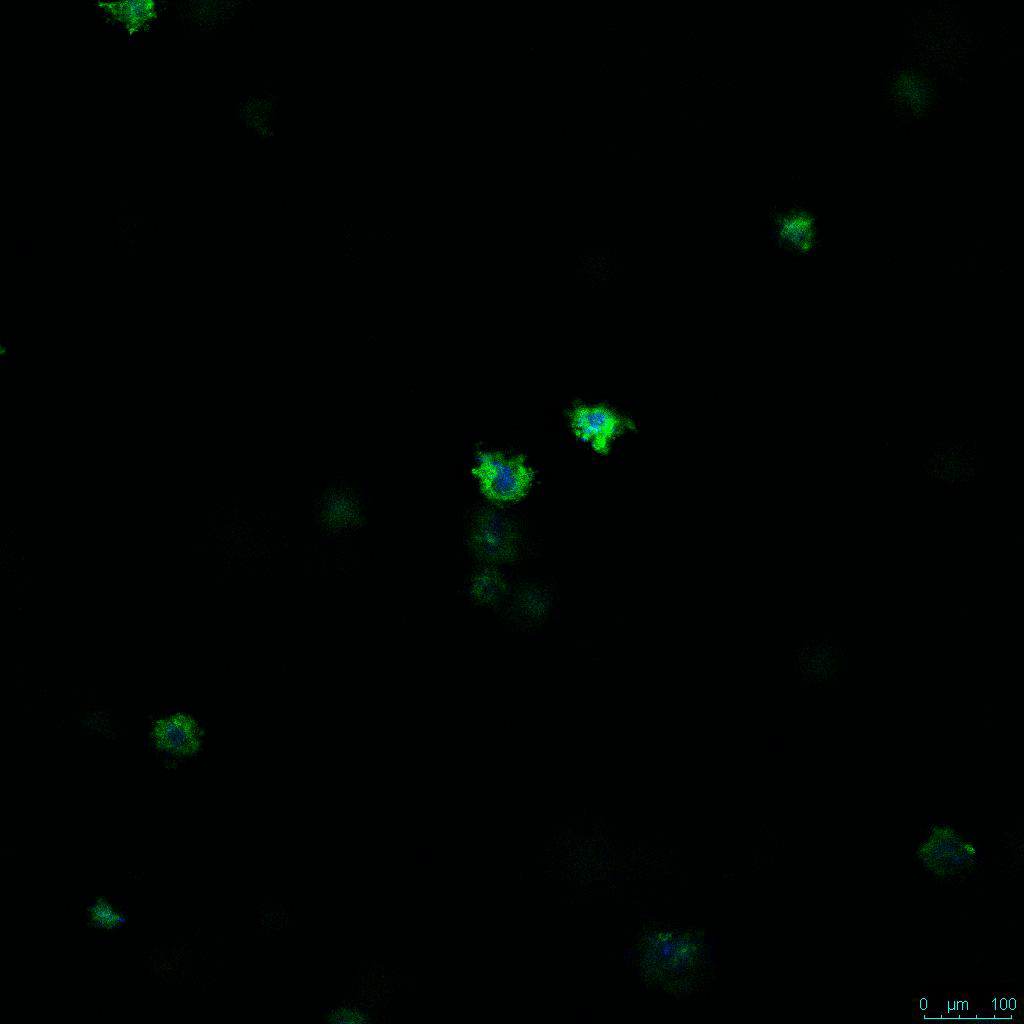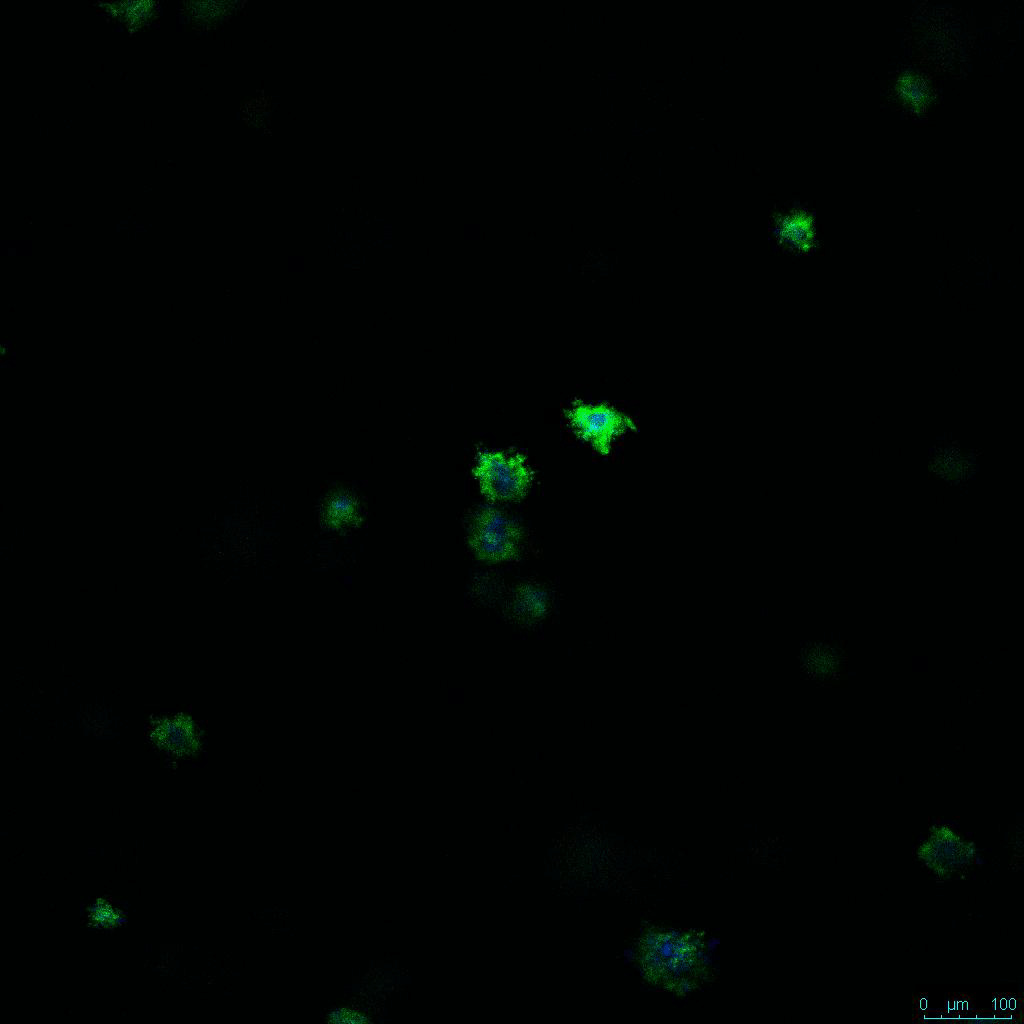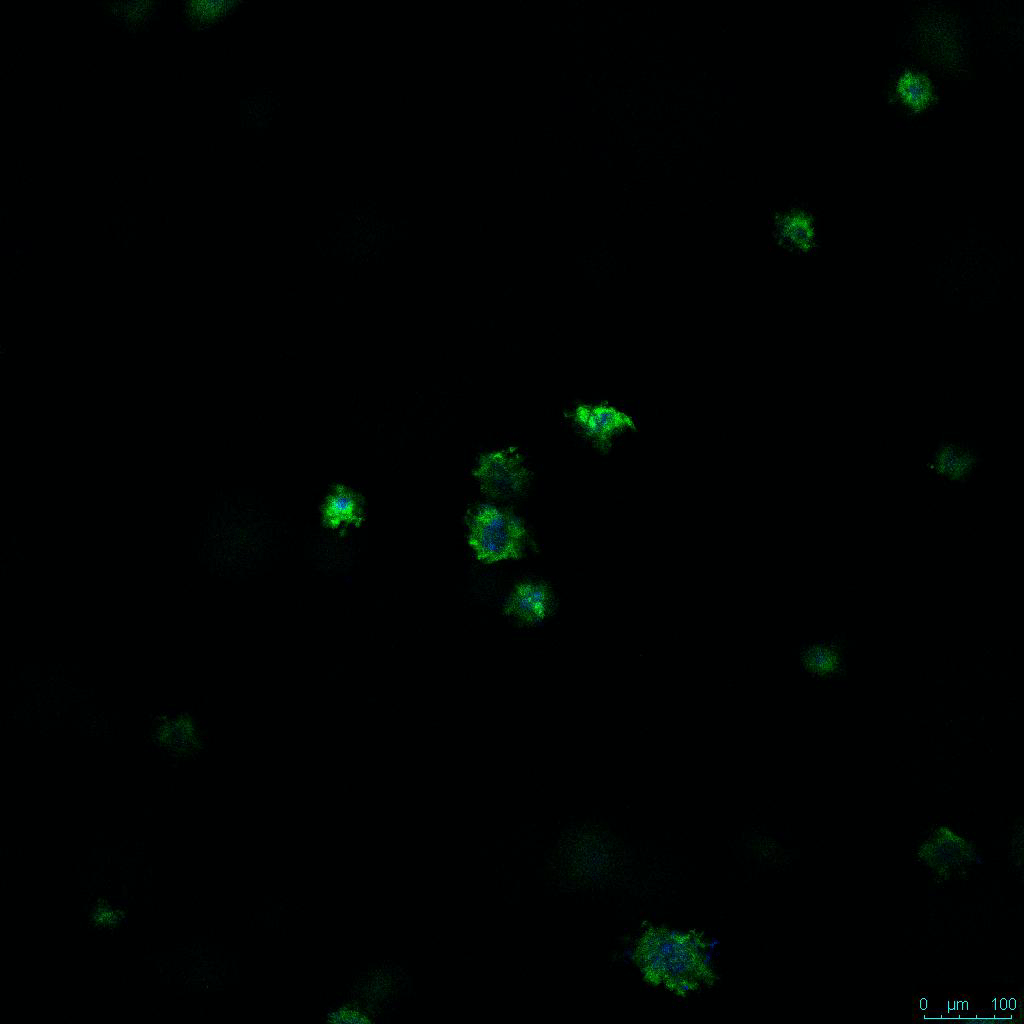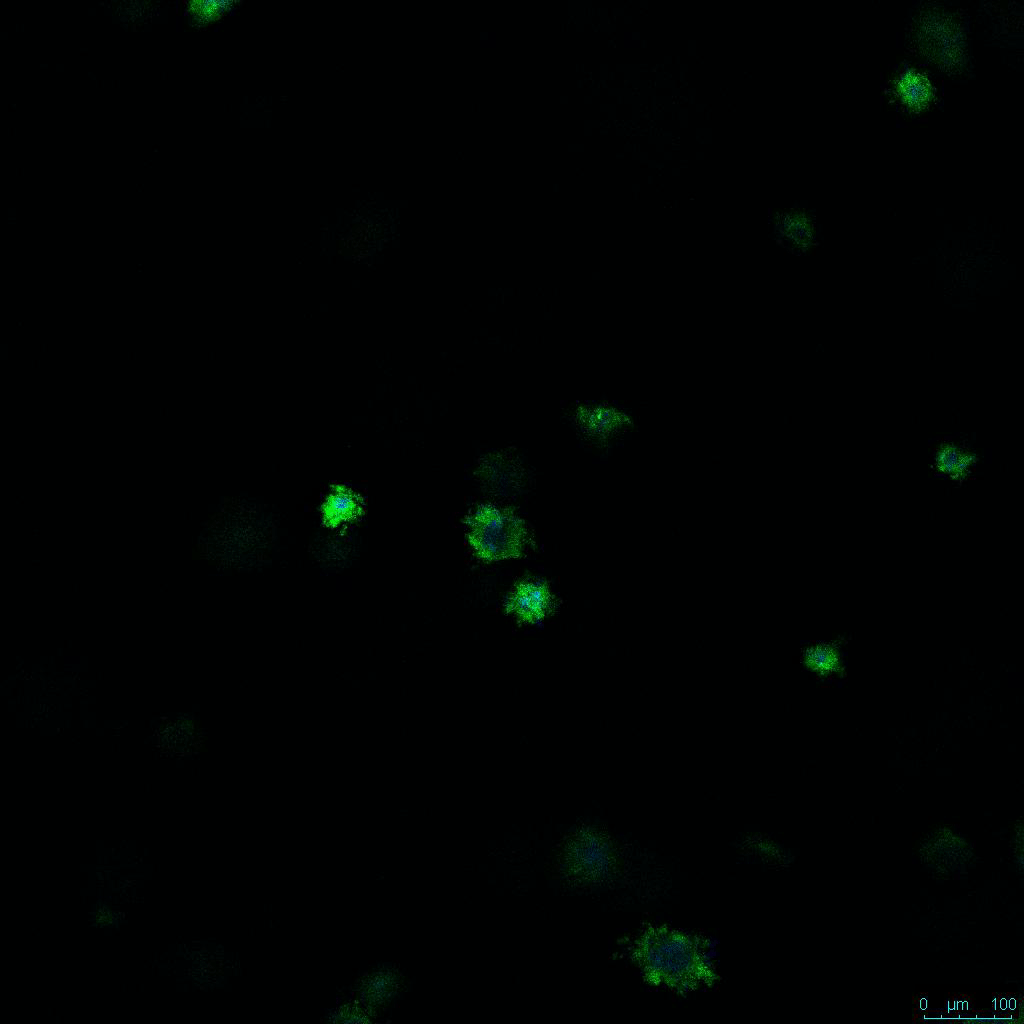
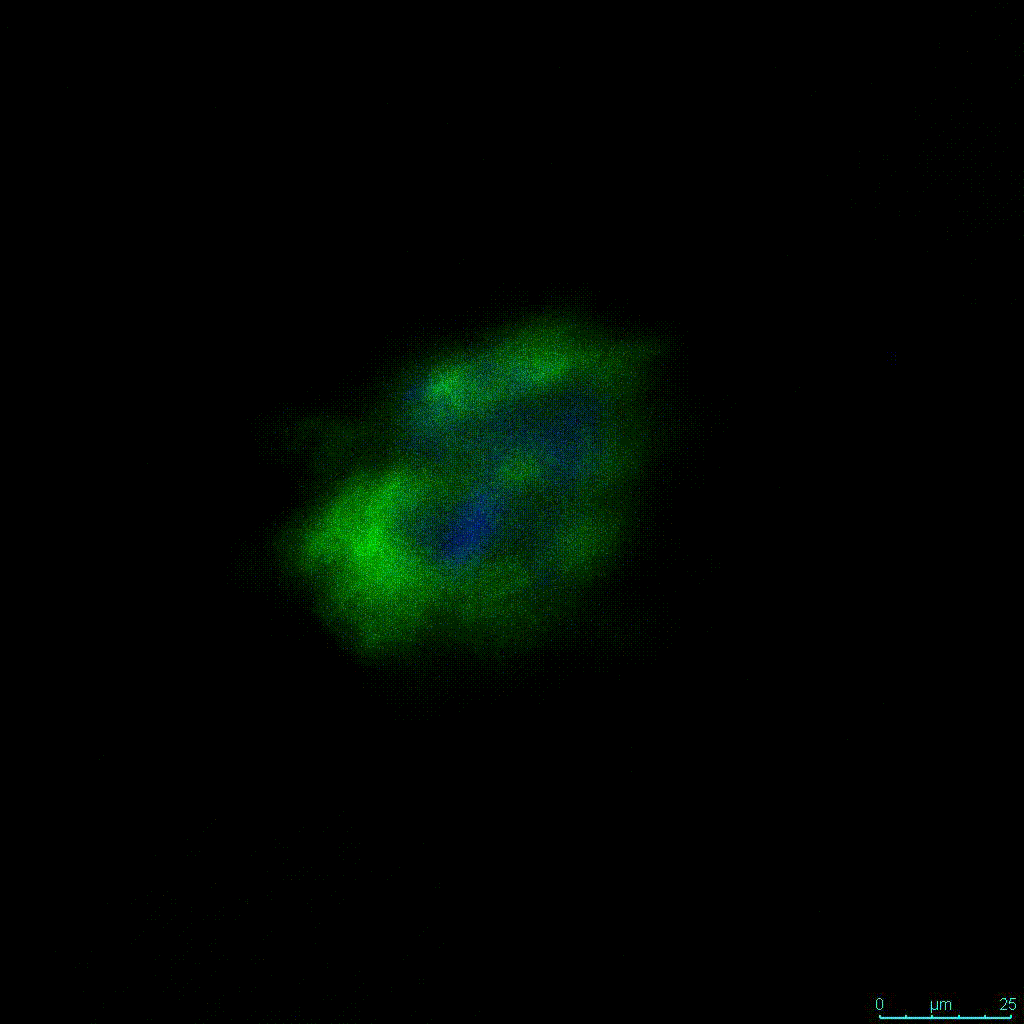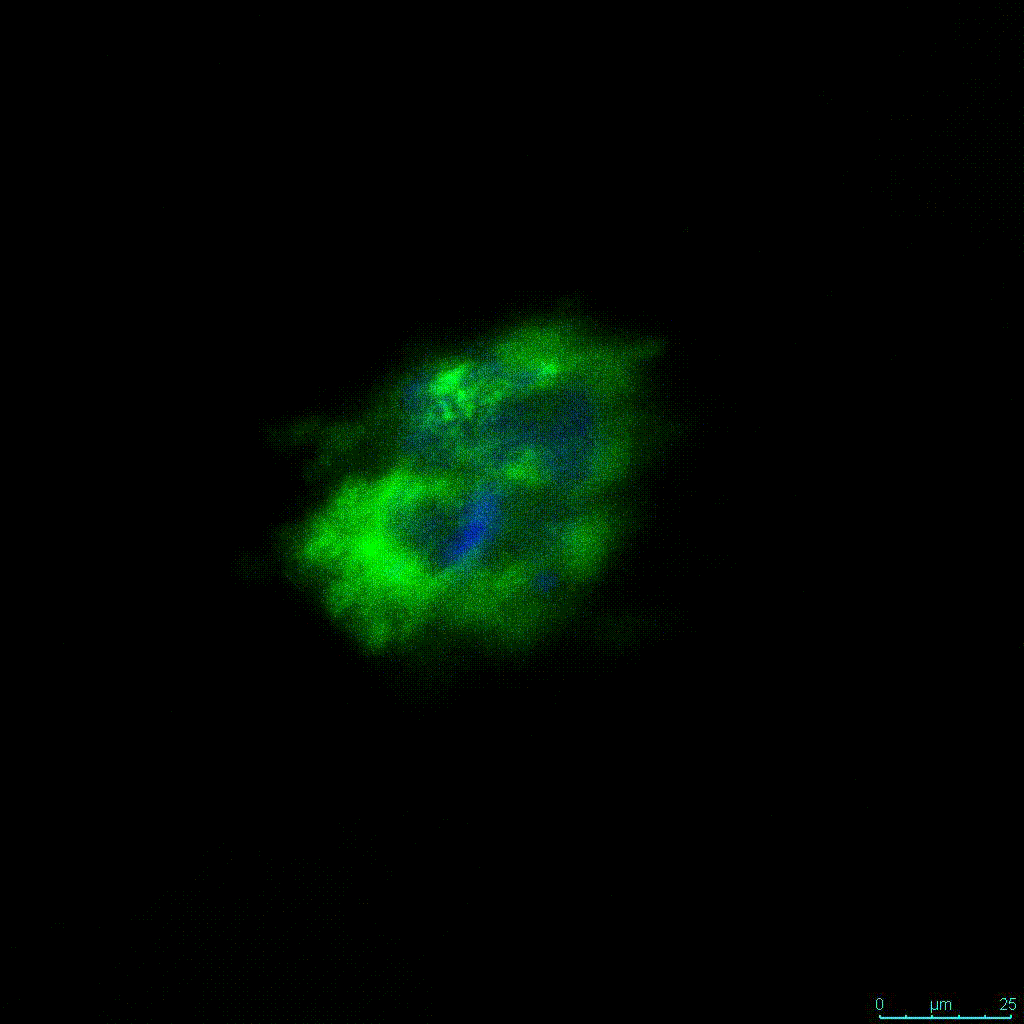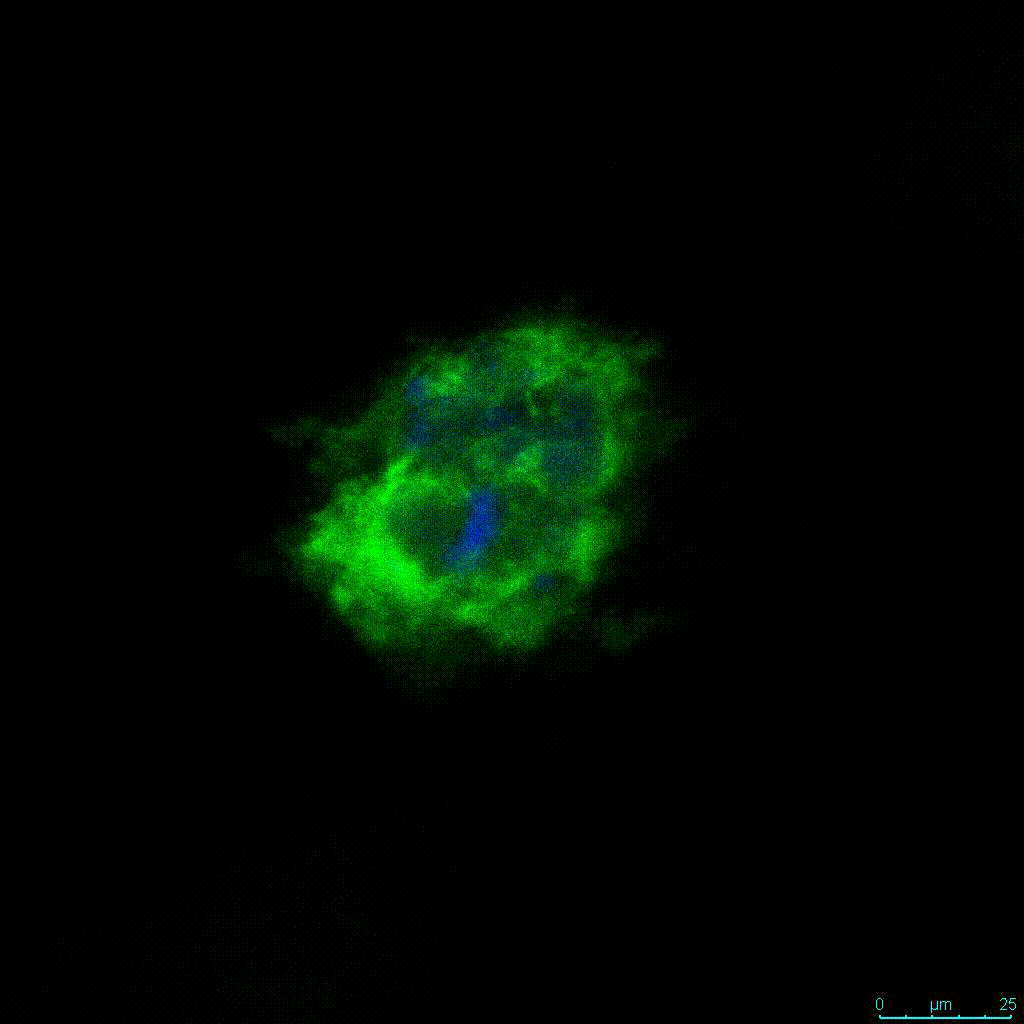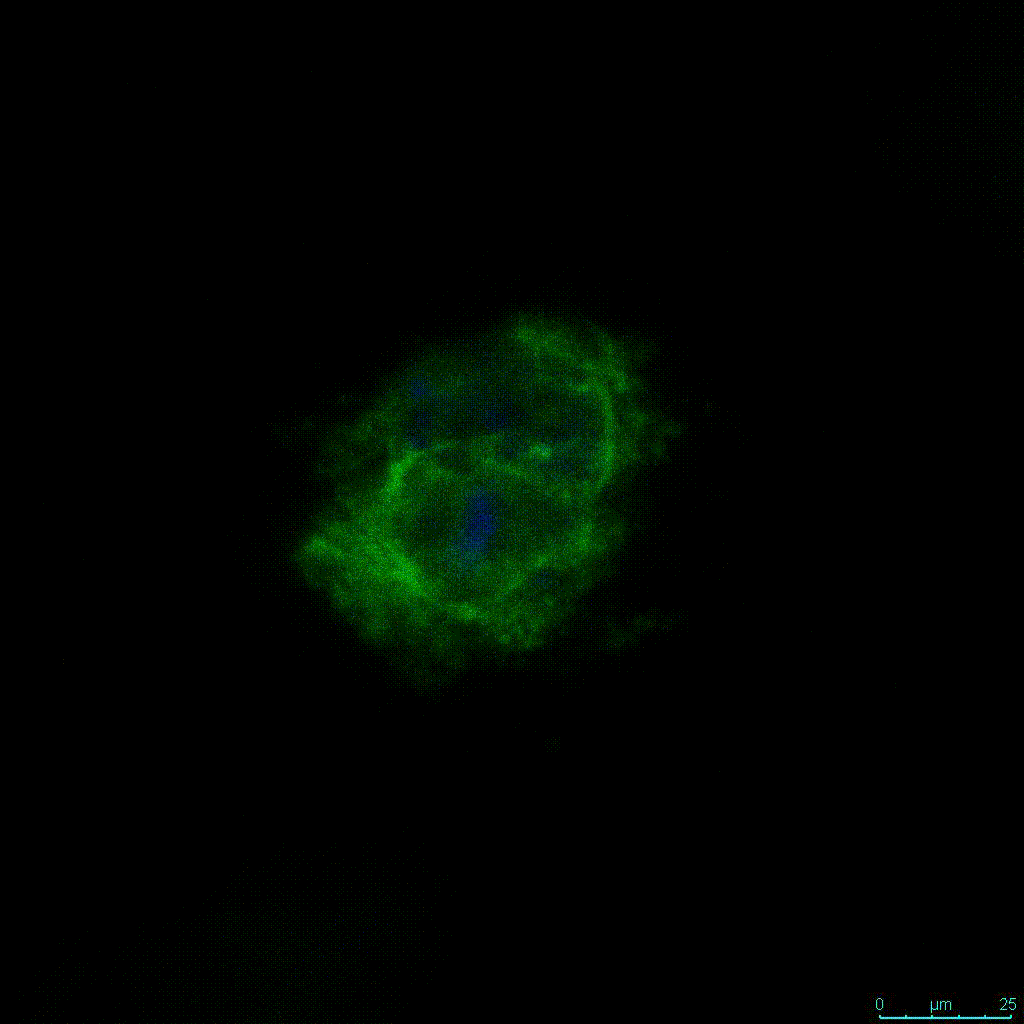
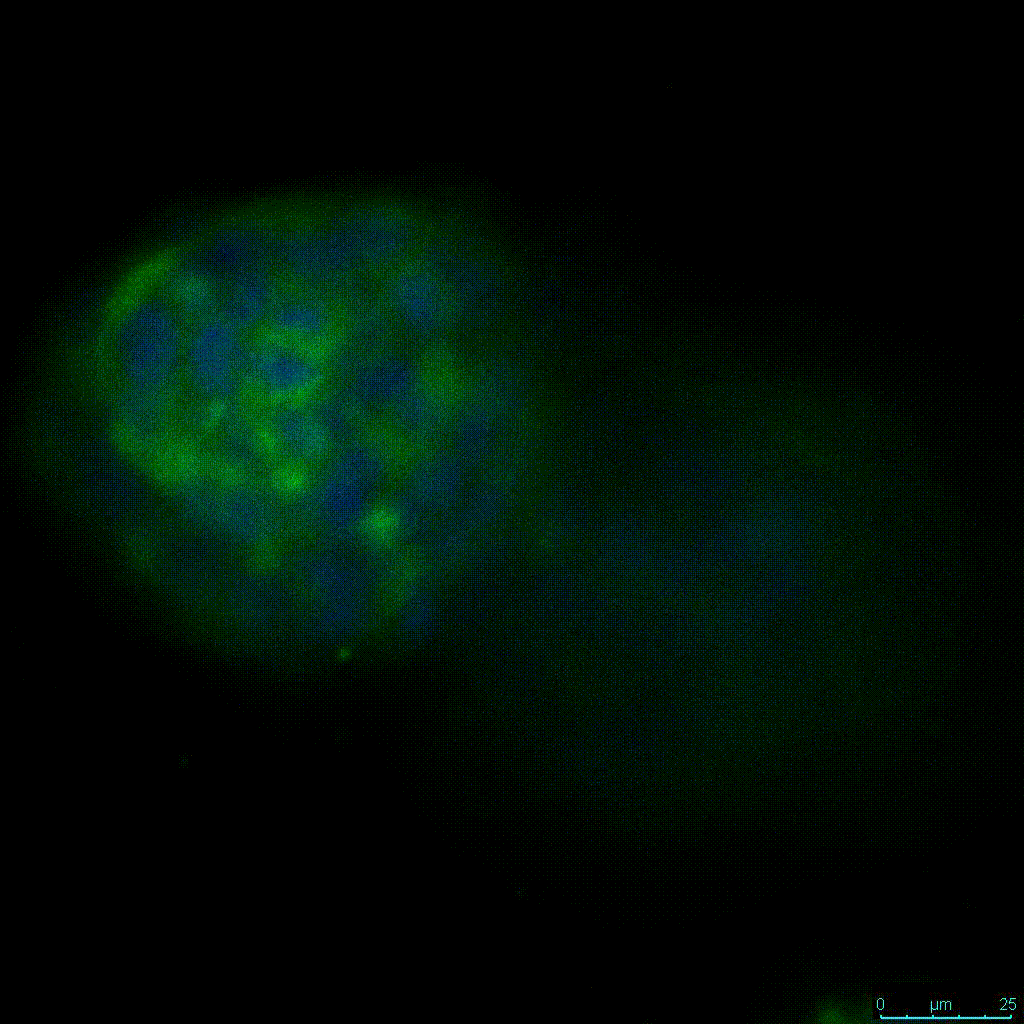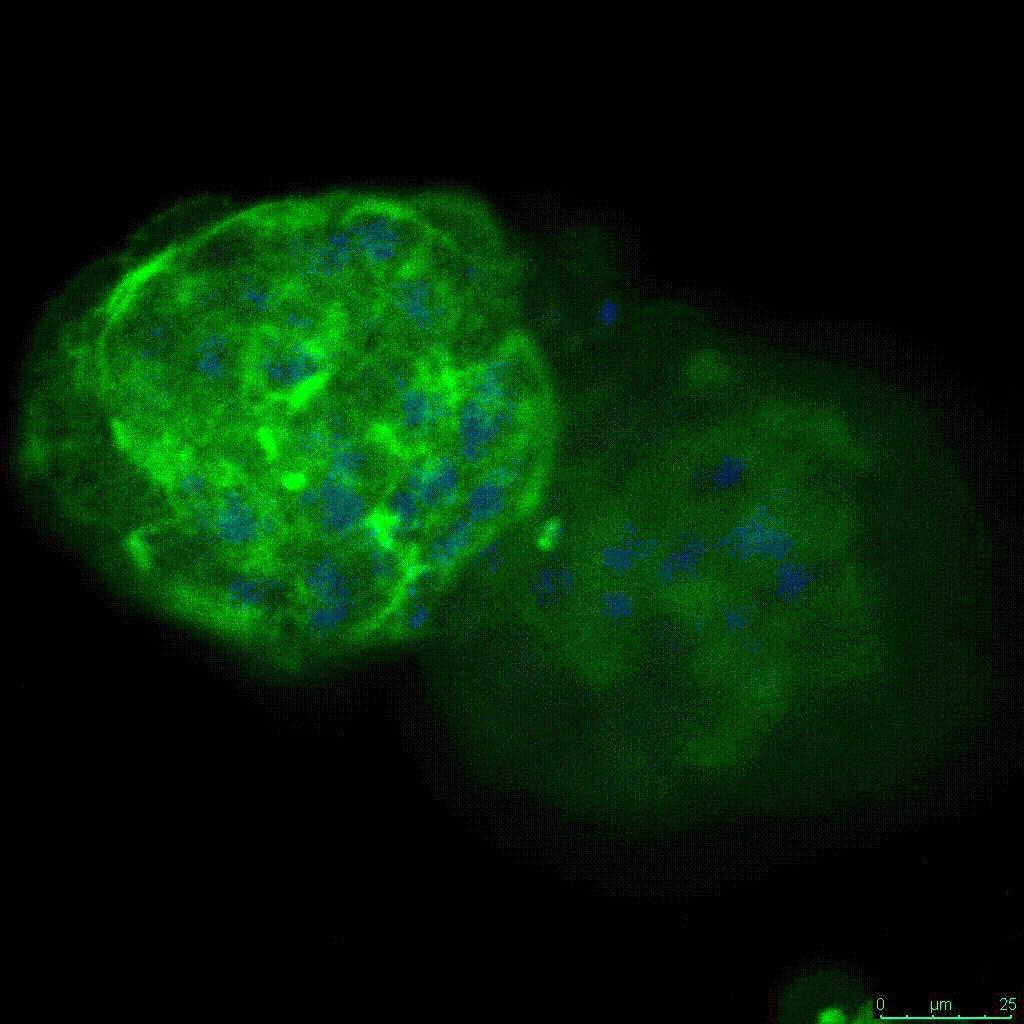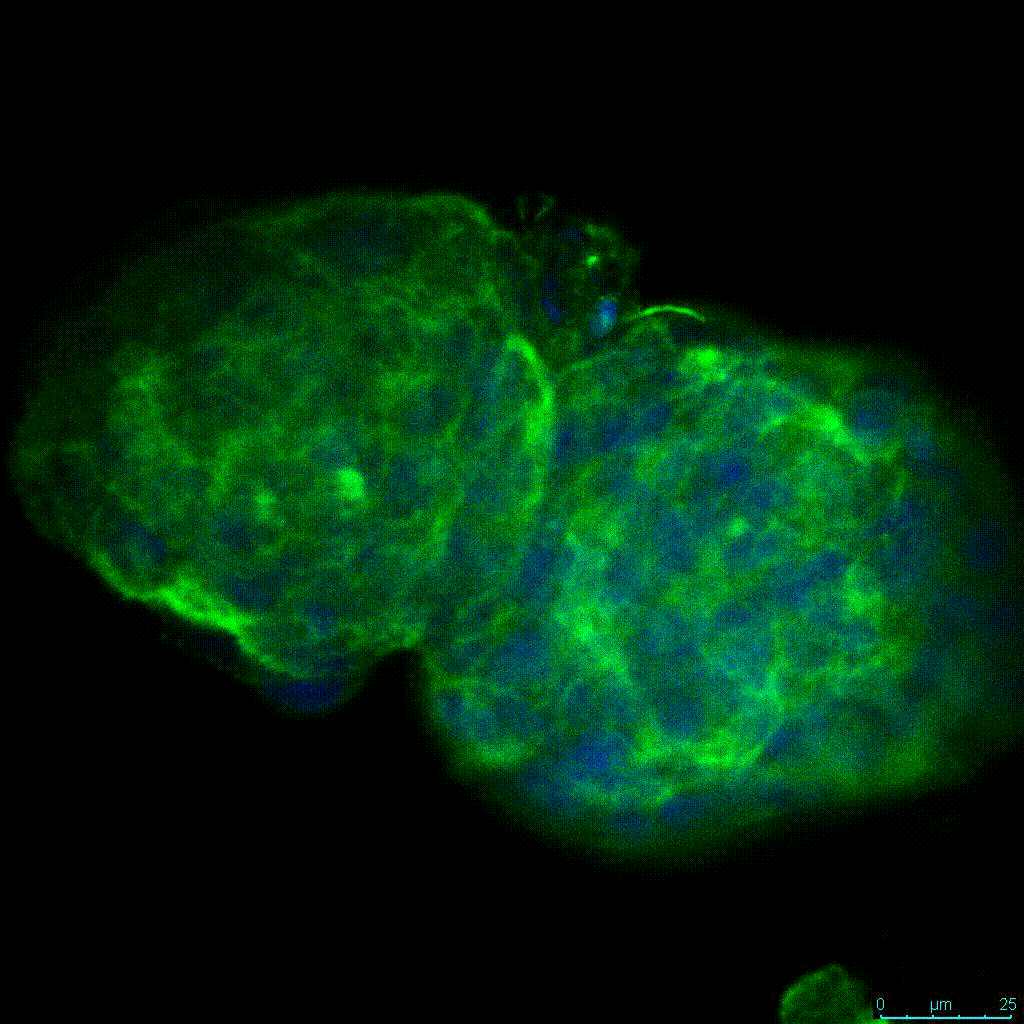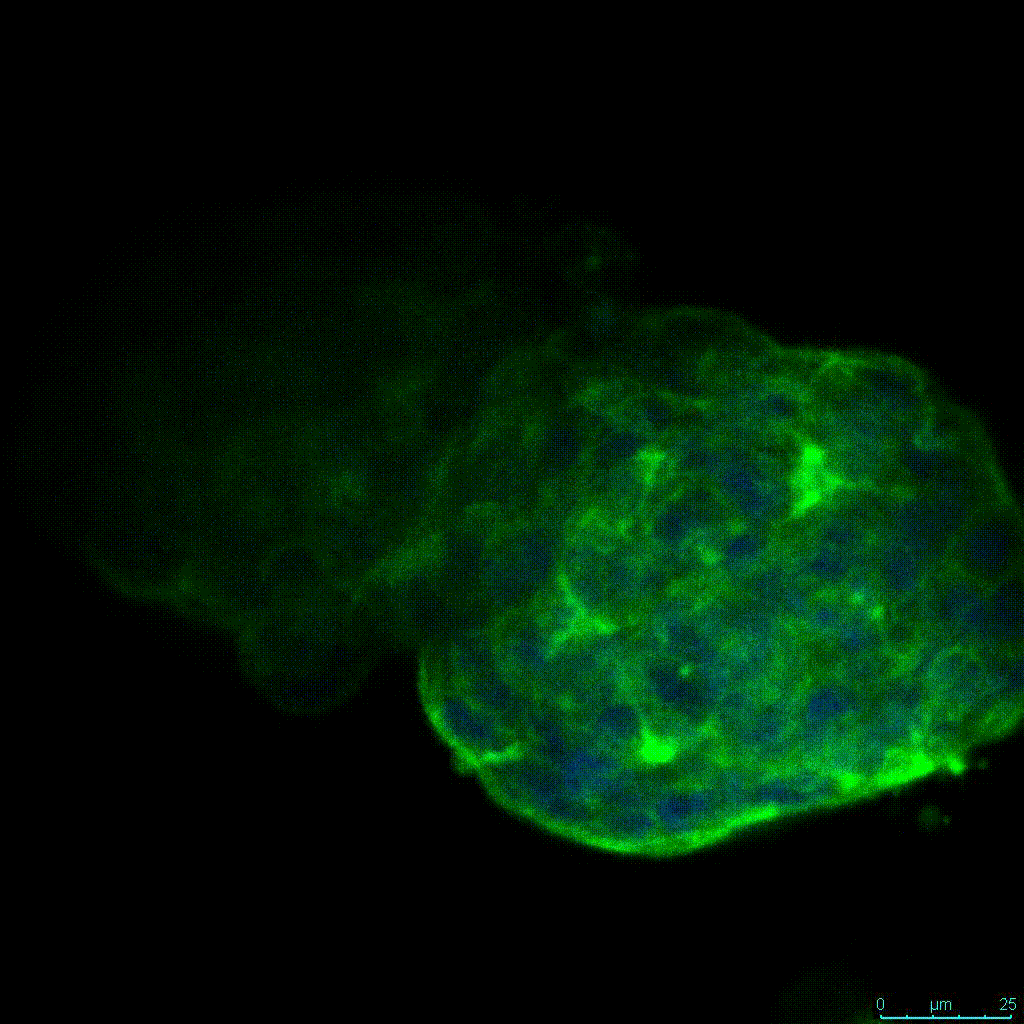
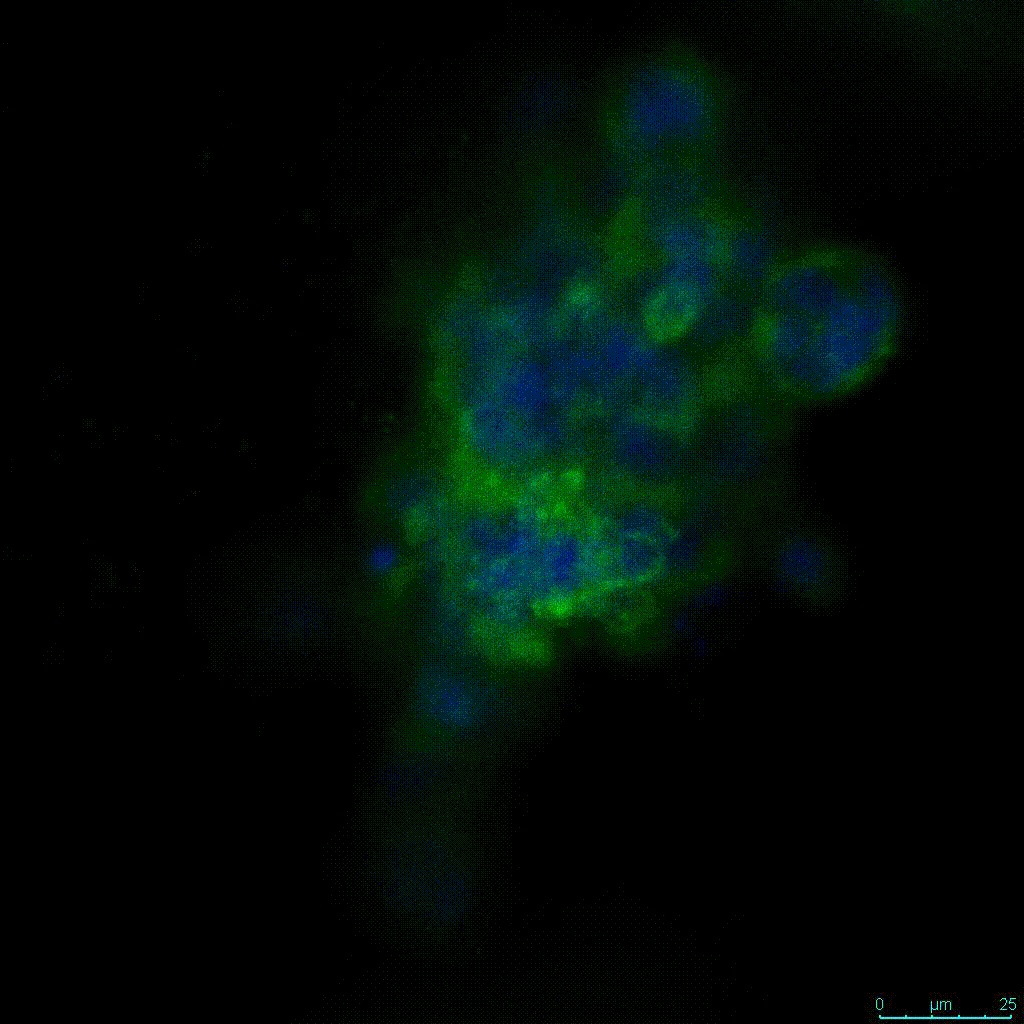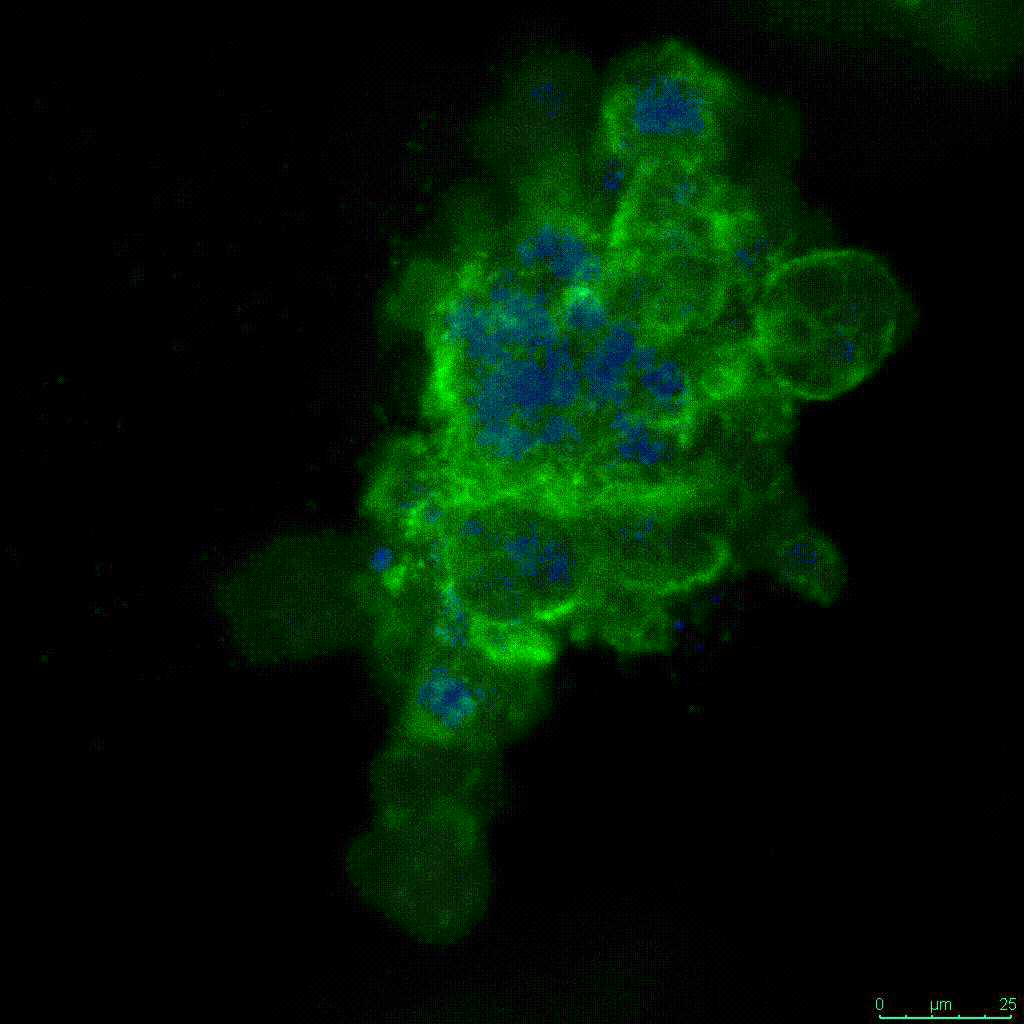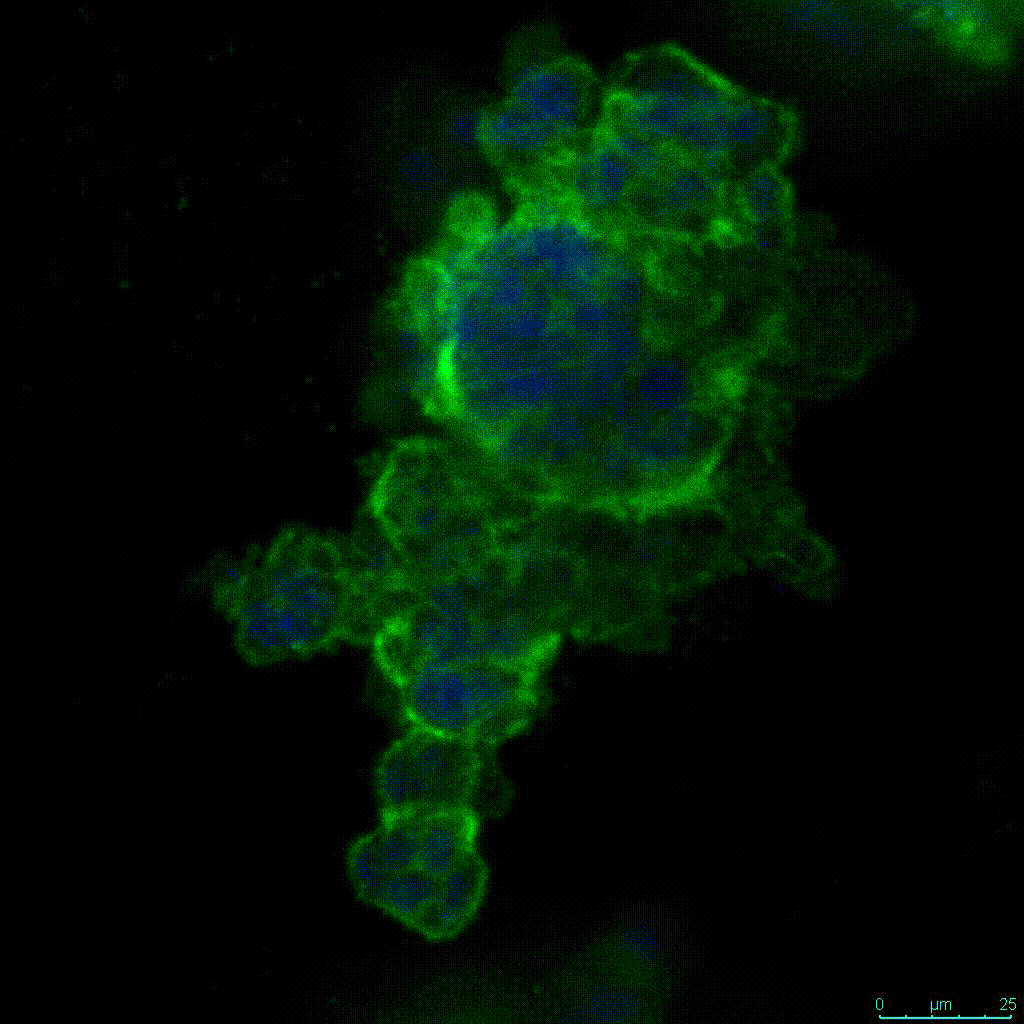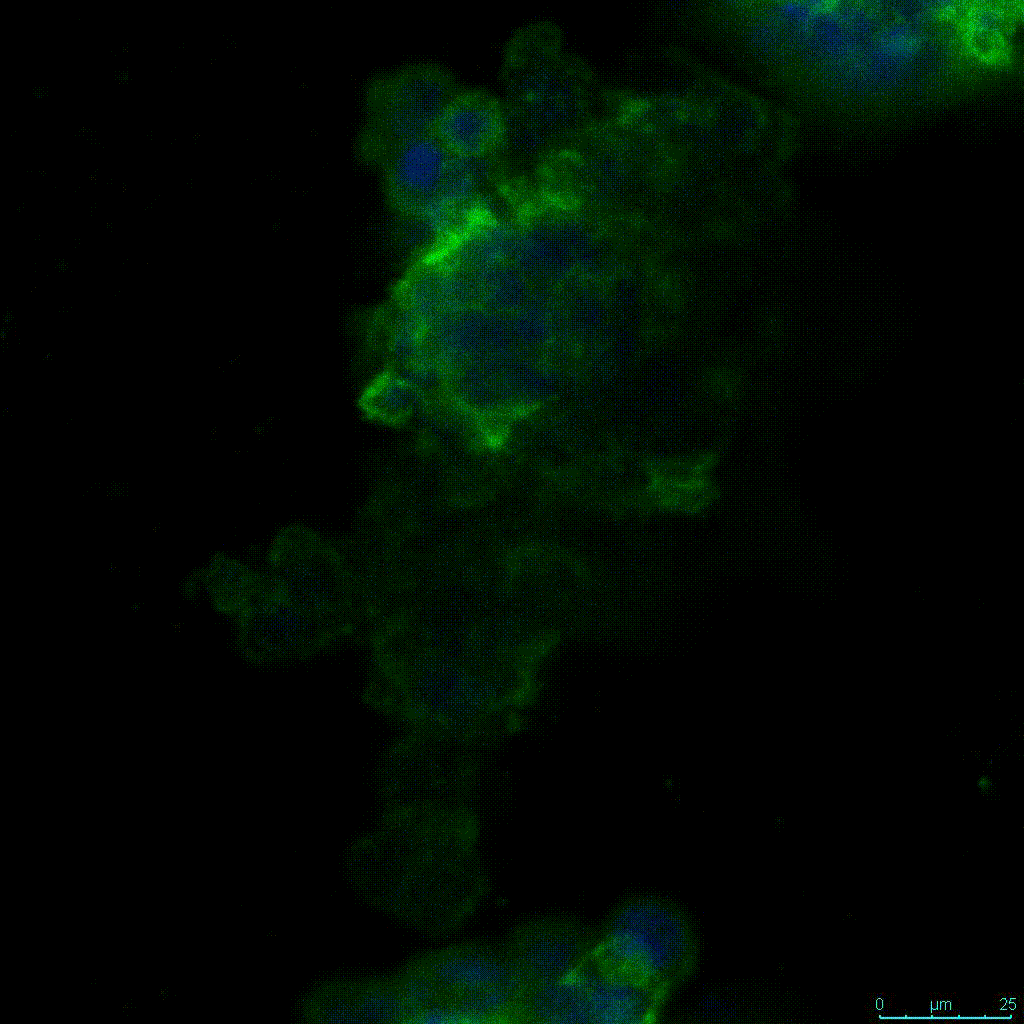
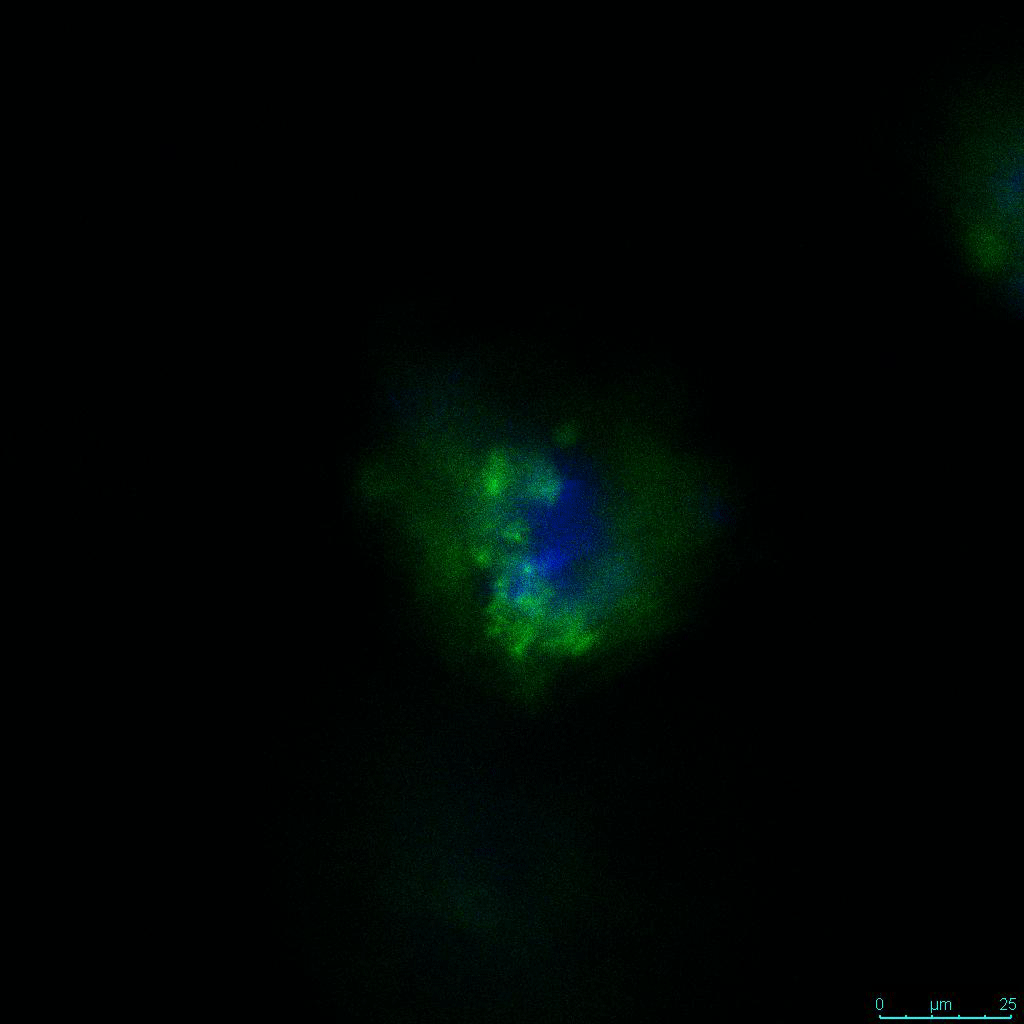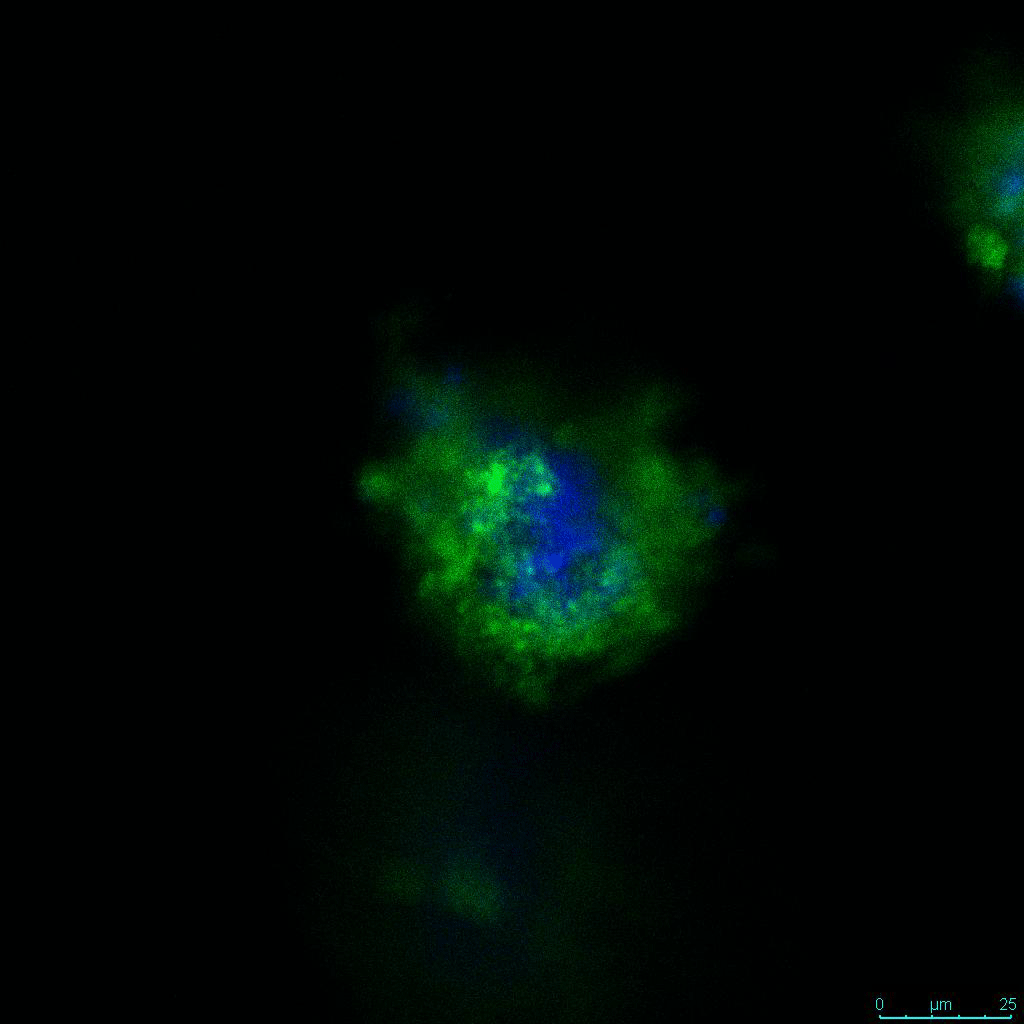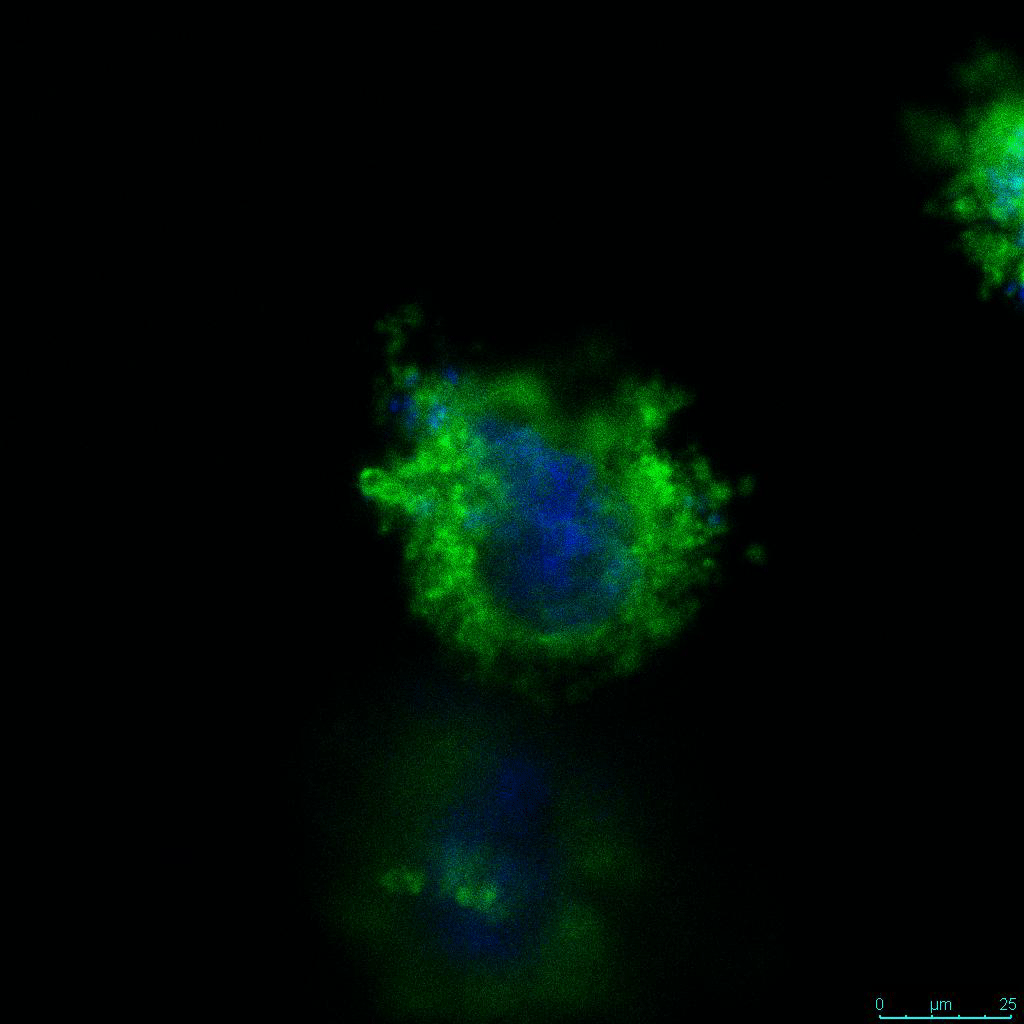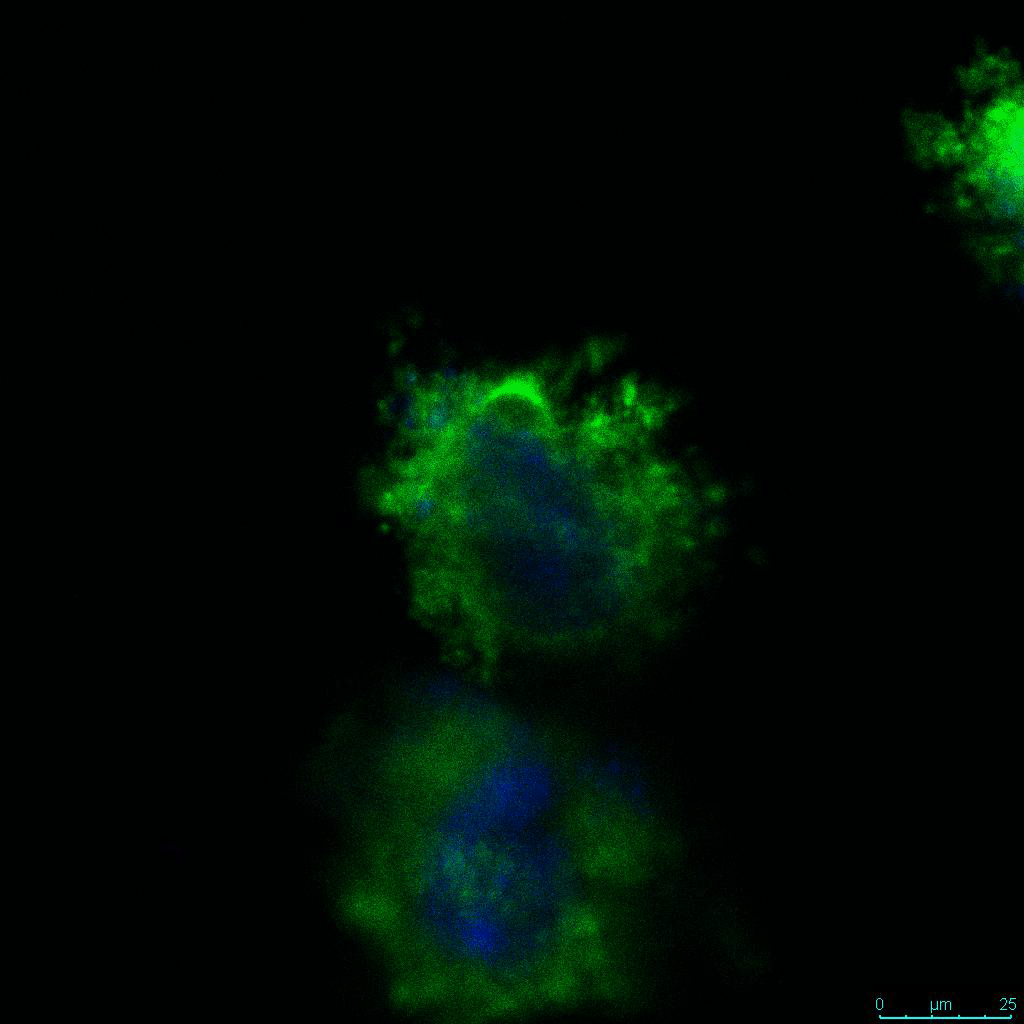
Figure 2. Videos
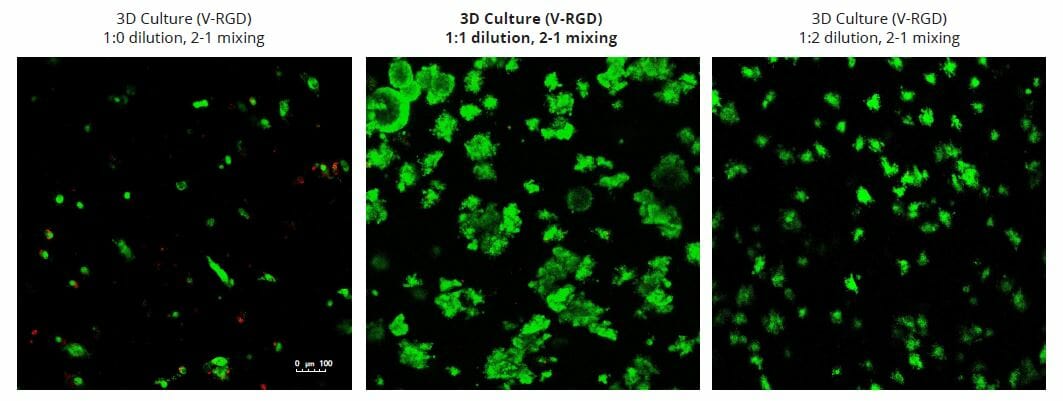
Figure 3: Cell viability of HCT116 cells in different hydrogel strengths.
VitroGel Culture of HTC116 Cells With 5-fluorouracil Show No Significant Response
5-fluorouracil (5-FU) is a common anti-cancer drug used to treat several cancers including colorectal cancer. While it has demonstrated some success in colon cancer, several more recent studies have pointed out that many patients develop a resistance to 5-FU (10), and other studies have found that 5-FU only acts on a small subset of colon cancers that have a mismatch repair gene mutation (11). Testing 5-FU on HCT116 cells using traditional 2D cell culture system juxtaposed against the 3D culture system demonstrated that the effect of the 5-FU is insignificant (Figure 4). We found that in 2D cultures, drug efficiency increased as the drug concentration increased from 10 µM to 50 µM. However, in the 3D cell culture system, there was no significant drug effect at any stage. Only in the case of 50 µM is there any noticeable effect, reduced size of cell colonies clearly observable. Quantification of cell death with a live/dead assay showed increasing levels of dead cells in the 2D cultures, suggesting an effectiveness of 5-FU in killing cancer cells. However, in 3D culture, there was not a significant level of dead cells at any stage; nearly non-existent until 50 µM. Quantification of live cells using ImageJ showed a steady decline with increased drug concentration. However, in 3D cultures, this decline was not present.
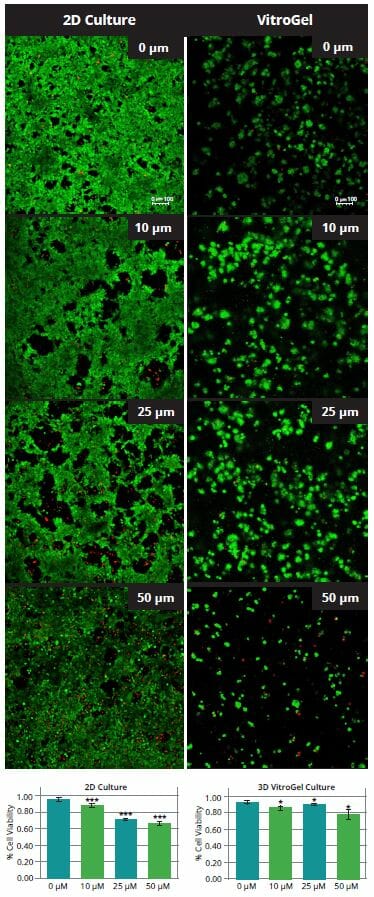
Figure 4. Cell viability of HCT116 cells following treatment with 5-FU at different concentrations. P-values less than 0.05 are considered to be significant (*), p-values less than 0.005 are very statistically significant (**) and values less than 0.001 are extremely statistically significant (***)
Discussion
Based on these results, we conclude that VitroGel-3D-RGD provides a viable alternative to traditional 2D culture for drug studies. Although the 2D coating culture on the standard VitroGel system also adopts a spheroid morphology, colonies are formed mainly through the aggregation of cell clusters instead of dividing from a single cell. Spheroids formed via 2D culture spheroids are also quite a bit larger than those derived from 3D culture, which may make it difficult for nutrients and drugs to permeate beyond the outer cell layer.
In 3D cell culture, we studied the cell morphology, growth, and survival in response to different hydrogel strengths (hydrogel dilution). We found that there is an optimal gel strength for HCT116 cells to grow: equal parts 0.5X PBS with hydrogel. The results of the live/dead assay showed that there was not an increase in cell death, suggesting that the reduction in the total number of cells in the lower and higher hydrogel dilutions resulted from the mechanical interactions of the cells with the hydrogel. Previous studies have found that the number of live cells is dependent upon substrate stiffness, while the number of dead cells is unrelated, suggesting that the stiffness of the hydrogel can affect proliferation (12, 13). In fact, studies conducted on hepatocellular carcinoma cells demonstrated that the mechanical properties of a matrix could not only promote proliferation but also can moderate chemotherapeutic response (14).
In the videos (above) related to Figure 2, we observed varying levels of Actin in different parts of the clusters and even between clusters in different 3D scaffold stiffness. Some clusters have more Actin than others, and Actin localization in scaffolds of different stiffness varies. Although we used Actin simply to observe cluster morphology (i.e. flat vs. spherical), it should be noted that there are studies that show that actin levels can fluctuate in cancer cells at different stages (15). In research, Actin is useful as a morphology marker because it is a homeostatic gene, but its actual function relates to multiple cellular processes from contraction to cell division and motility. One study found that actin filaments located at the leading edge of cancer cells are motile in nature and have a higher rate of turnover (16). Another study found that actin plays a major role in both pre-malignant and malignant breast tumor cells by first controlling cell stiffness to promote proliferation, then cell softness to allow for invasion (17). The variation in Actin localization and expression levels between different scaffold rigidities concomitant with studies that show that Actin expression varies in cancer cells suggests that the 3D scaffold can affect the motility of the cell.
Because cells respond uniquely to matrix rigidity, it is likely that each cell line will have to be optimized prior to experimentation. However, the wide array of flexibility this system offers with regard to culture medium compatibility and its potential to be used in vitro and in vivo make VitroGel a great option for drug testing, especially for tumor applications.
Reference
- Fang Y, Eglen R. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS DISCOVERY: Advancing Life Sciences R&D. 2017;22(5):456-472.
- Langhans S. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front Pharmacol. 2018;22(5):456–472.
- Edmondson R, Broglie J, Adcock A, Yang L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev Technol. 2014;12(4):207-218.
- Kloxin A, Kloxin C, Bowman C, Anseth K. Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination. Advanced Materials. 2010;22(31):3484-3494.
- Zanoni M, Piccinini F, Arienti C et al. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep. 2016;6(1).
- Mahauad-Fernandez W, Okeoma C. B49, a BST-2-based peptide, inhibits adhesion and growth of breast cancer cells. Sci Rep. 2018;8(1).
- Powell K. Adding depth to cell culture. Science | AAAS. http://www.sciencemag.org/features/2017/04/adding-depth-cell-culture. Published 2017. Accessed August 13, 2018.
- VitroGel. Thewellbio.com. https://www.thewellbio.com/wp-content/uploads/2018/05/VitroGel-3D-Protocol-Guidelines.pdf. Published 2018. Accessed August 13, 2018.
- Bellis S. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials. 2011;32(18):4205-4210.
- Bracht K, Nicholls A, Liu Y, Bodmer W. 5-Fluorouracil response in a large panel of colorectal cancer cell lines is associated with mismatch repair deficiency. Br J Cancer. 2010;103(3):340-346.
- He J, Pei L, Jiang H, Yang W, Chen J, Liang H. Chemoresistance of colorectal cancer to 5-fluorouracil is associated with silencing of the BNIP3 gene through aberrant methylation. J Cancer. 2017;8(7):1187-1196.
- Cavo M, Fato M, Peñuela L, Beltrame F, Raiteri R, Scaglione S. Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Sci Rep. 2016;6(1).
- Cook J, Fox D, Kuroki K, Jayo M, De Deyne P. In vitro and in vivo comparison of five biomaterials used for orthopedic soft tissue augmentation. Am J Vet Res. 2008;69(1):148-156.
- Schrader J, Gordon-Walker T, Aucott R et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53(4):1192-1205.
- Yamaguchi, Hideki, and John Condeelis. “Regulation Of The Actin Cytoskeleton In Cancer Cell Migration And Invasion”. Biochimica Et Biophysica Acta (BBA) – Molecular Cell Research, vol 1773, no. 5, 2007, pp. 642-652.
- Tavares, Sandra et al. “Actin Stress Fiber Organization Promotes Cell Stiffening And Proliferation Of Pre-Invasive Breast Cancer Cells”. Nature Communications, vol 8, 2017, p. 15237.
- Lorente, Gisela et al. “Actin Filaments At The Leading Edge Of Cancer Cells Are Characterized By A High Mobile Fraction And Turnover Regulation By Profilin I”. Plos ONE, vol 9, no. 1, 2014, p. e85817.